

Chinese Journal of Organic Chemistry >
A Benzothiazole-Derived Fluorescence Enhancement Probe for Visual Detection of H2S
Received date: 2014-04-09
Revised date: 2014-05-05
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 51173060).
A benzothiazole-derived fluorescence enhancement probe (FL) for the detection of H2S was synthesized and characterized by employing azide group as the recognition unit, and its spectral properties had been researched. The results showed that FL exhibited relatively good sensitivity and selectivity to H2S as well as fast response, it also had strong anti-interference ability and low cytotoxicity, the detection limit of H2S was estimated to be 8.78×10-7 mol·L-1, and the detection of H2S in living HeLa cells and various water samples indicated that it had potential application value.
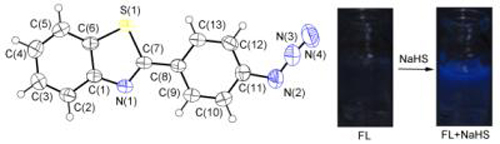
Key words: H2S; fluorescent probe; azide group; visualization
Fan Fanglu , Jing Jinqiu , Chen Xuemei . A Benzothiazole-Derived Fluorescence Enhancement Probe for Visual Detection of H2S[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 2178 -2183 . DOI: 10.6023/cjoc201404015
[1] Li, L.; Rose, P.; Moore, P. K. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169.
[2] Kabil, O.; Banerjee, R. J. Biol. Chem. 2010, 285, 21903.
[3] Lefer, D. J. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17907.
[4] Blackstone, E.; Morrison, M.; Roth, M. B. Science 2005, 308, 587.
[5] Huang, B.; Chen, Y.-C.; Guo, Z.-J.; He, W.-J. Chin. J. Inorg. Chem. 2013, 29, 2283 (in Chinese).
(黄彬, 陈韵聪, 郭子建, 何卫江, 无机化学学报, 2013, 29, 2283.)
[6] Liu, J.; Sun, Y.; Zhang, J.; Yang, T.; Cao, J.; Zhang, L.; Guo, W. Chem. Eur. J. 2013, 19, 4717.
[7] Kumar, N.; Bhalla, V.; Kumar, M. Coord. Chem. Rev. 2013, 257, 2335.
[8] Jiang, Y.; Wu, Q.; Chang, X. Talanta 2014, 121, 122.
[9] Zhang, J.; Guo, W. Chem. Commun. 2014, 50, 4214.
[10] Mao, G.; Wei, T.; Wang, X.; Huan, S.; Lu, D.; Zhang, J.; Zhang, X.; Tan, W.; Shen, G.; Yu, R. Anal. Chem. 2013, 85, 7875.
[11] Chen Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. Angew. Chem., Int. Ed. 2013, 52, 1688.
[12] Wang, X.; Sun, J.; Zhang, W.; Ma, X.; Lv, J.; Tang, B. Chem. Sci. 2013, 4, 2551.
[13] Lippert, A. R.; New, E. J.; Chang, C. J. J. Am. Chem. Soc. 2011, 133, 10078.
[14] Peng, H.; Cheng, Y.; Dai, C.; King, A. L.; Predmore, B. L.; Lefer, D. J.; Wang, B. Angew Chem., Int. Ed. 2011, 50, 9672.
[15] Montoya, L. A.; Pluth, M. D. Chem. Commun. 2012, 48, 4767.
[16] Zhou, G.; Wang, H.; Ma, Y.; Chen, X. Tetrahedron 2013, 69, 867.
[17] Li, W.; Sun, W.; Yu, X.; Du, L.; Li M. J. Fluoresc. 2013, 23, 181.
[18] Wan, Q.; Song, Y.; Li, Z.; Gao, X.; Ma, H. Chem. Commun. 2013, 49, 502.
[19] Hartman, M. C. T.; Dcona. M. M. Analyst 2012, 137, 4910.
[20] Yu, F.; Li, P.; Song, P.; Wang, B.; Zhao, J.; Han, K. Chem. Commun. 2012, 48, 2852.
[21] Huang, Q.; Yang, X.; Li, H. Dyes Pigm. 2013, 99, 871.
[22] Yang, Y.; Li, B.; Zhang, L.; Guan, Y. J. Lumin. 2014, 145, 895.
[23] Shortreed M, Kopelman R, Kuhn M, Hoyland, B. Anal. Chem. 1996, 68, 1414.
[24] Shi, D. F.; Bradshaw, T. D.; Wrigley, S.; McCall, C. J.; Lelieveld, P.; Fichtner, I.; Stevens, M. F. G. J. Med. Chem. 1996, 39, 3375.
[25] Sheldrick, G. M. SHELXS-97, Program for the Solution of Crystal Structures, University of Göttingen, Germany, 1997.
[26] Sheldrick, G. M. SHELXL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997.
[27] Liu, Y.; Fei, Q.; Shan, H.; Cui, M.; Liu, Q.; Feng, G.; Huan, Y. Analyst 2014, 139, 1868.
/
| 〈 |
|
〉 |