

Chinese Journal of Organic Chemistry >
Synthesis of 1-Hydroxy-2-phenyltriphenylene Derivatives by Three-Step Condensation
Received date: 2014-03-06
Revised date: 2014-03-27
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372150), the Natural Science Foundation of Shaanxi Province (No. 2014JQ2073) and the Fundamental Research Funds for the Central Universities (No. GK261001095).
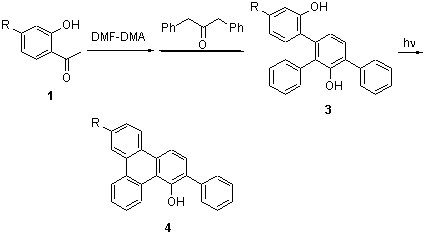
(E)-3-(Dimethylamino)-1-(2-hydroxyphenyl)prop-2-en-1-ones (2) were synthesized by the condensation reaction of o-hydroxyacetophenones (1) and N,N-dimethylformamide-dimethyl acetal (DMF-DMA). Using DMF as solvent and K2CO3 as base, 4'-phenyl-(1,1':2',1''-terphenyl)-2,3'-diol (3) were synthesized via the condensation reaction of intermediate 2 and 1,3-diphenylacetones. 3 were dissolved in the solution of acetonitrile-1% HCl (V:V=1:1) and irradiated by 500 W mercury lamp, and 1-hydroxy-2-phenyltriphenylenes were obtained. This process to synthesize 4 has the advantages of simple operation, high efficiency of the atomic, without oxidant and catalyst. Compounds 3 and 4 were confirmed by the method of FT-IR, 1H NMR, 13C NMR, HRMS.
Wang Wenli , He Qing , Ding Ru , He Yun , Zhang Zunting . Synthesis of 1-Hydroxy-2-phenyltriphenylene Derivatives by Three-Step Condensation[J]. Chinese Journal of Organic Chemistry, 2014 , 34(9) : 1875 -1880 . DOI: 10.6023/cjoc201405008
[1] Primi, D.; Fiordalisi, G.; Mantero, J.L.; Mattioli, S.; Sottini, A.; Bonelli, F.; Vaglini, L.WO 0028039, 2000 [Chem.Abstr.2000, 133, 1451].
[2] Schulte, J.L.; Laschat, S.; Vill, V.; Nishikawa, E.; Finkelmann, H.; Nimtz, M.Eur.J.Org.Chem.1998, 2499.
[3] Breslow, R.; Jaun, B.; Kluttz, R.Q.; Xia, C.Z.Tetrahedron 1982, 6, 863.
[4] Fontes, E.; Heiney, P.A.; de Jeu, W.H.Phys.Rev.Lett.1988, 61, 1202.
[5] Schmidt-Mende, L.; Fechtenkötter, A.; Müllen, K.; Moons, E.; Friend, R.H.; MacKenzie, J.D.Science 2001, 293, 1119.
[6] Kawano, M.; Kawamichi, T.; Haneda, T.; Kojima,T.; Fujita, M.J.Am.Chem.Soc.2007, 50, 15418.
[7] Wettach, H.; Jester, S.S.; Colsmann, A.; Lemmer, U.; Rehmann, N.; Meerholz, K.; Höger, S.Synth.Met.2010, 7~8, 691.
[8] Huang, C.-J.M.S.Thesis, Tsinghua University, Xinzhu, Taiwan, 2008 (in Chinese).(黄崇杰, 硕士论文, 国立清华大学, 新竹, 台湾, 2008.)
[9] Li, Z.-W.M.S.Thesis, Tianjin University, Tianjin, 2012 (in Chinese).(李志伟, 硕士论文, 天津大学, 天津, 2012.)
[10] Liu, J.-S.M.S.Thesis, Anhui Normal University, Wuhu, 2003 (in Chinese).(刘金水, 硕士论文, 安徽师范大学, 芜湖, 2003.)
[11] Mao, X.-H.; He, Z.-Q.; Zhang, C.-X.Chin.J.Org.Chem.2006, 26, 413 (in Chinese).(毛华香, 何志群, 张春秀, 有机化学, 2006, 26, 413.)
[12] Cammidge, A.N.; Bushby, R.J.Hand Book of Liquid Crystals, Vol.2B, New York, 1998, Chapter VII.
[13] Kumar, S.; Manickam, M.Chem.Commun.1997, 17, 1615.
[14] Kumar, S.; Varshney, S.K.Synthesis 2001, 0305.
[15] Iwasaki, M.; Iino, S.; Nishihara, Y.Org.Lett.2013, 20, 5326.
[16] Pena, D.; Escudero, S.; Perez, D.; Guitian, E.; Castedo, L.Angew.Chem., Int.Ed.1998, 37, 2659.
[17] García-López, J.A, Greaney, M.F.Org.Lett.2014, 9, 2338.
[18] Le Berre, V.; Angely, L.; Simonet, J.; Mousset, G.; Bellec, M.J.Electroanal.Chem.Interf.Electrochem.1987, 1, 173.
[19] Freudemann, R.; Behnisch, B.; Hanack, M.J.Mater.Chem.2001, 11, 1618.
[20] Nayak, M.K.; Wan, P.Photochem.Photobiol.Sci.2008, 12, 1544.
[21] Biegasiewicz, K.F.; Denis, J.D.S.; Carroll, V.M.; Priefer, R.Tetrahedron Lett.2010, 33, 4408.
[22] Nairoukh, Z.; Fanun, M.; Schwarze, M.; Schomöcker, R.; Blum, J.J.Mol.Catal.A: Chem.2014, 382, 93.
/
| 〈 |
|
〉 |