

Chinese Journal of Organic Chemistry >
Synthesis and Characterization of a Visualization and Water-Soluble Fluorescent Probe for Al3+
Received date: 2014-03-08
Revised date: 2014-03-24
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372150), and the Fundamental Research Funds for the Central Universities (No. GK261001095).
In order to improve the water-solubility of 4-(2'-hydroxyphenyl)-pyrimido[1,6-a]benzimidazole and 4-(2'-hydrx- yphenyl-4'-isopropoxy)-pyrimido[1,6-a]benzimidazoles, they were sulfonated in 98% H2SO4. Sodium 4-(2'-hydroxyphenyl)- pyrimido[1,6-a]benzimidazole-5-sulfonate (L1), sodium 4-(2'-hydroxyphenyl)-pyrimido[1,6-a]benzimidazole-3',5',10'-three- sulfonate (L2), sodium 4-(2',4'-dihydroxyphenyl)pyrimido[1,6-a]benzimidazol-5-sulfonate (L3) were obtained and indentified by the method of NMR, IR and MS. Fluorescence spectra of L1, L2 and L3 were detected, and they have the ESIPT effect with a large Stokes shift (175~225 nm). The structures of these compounds could be complex with Al3+, and the fluorescence spectra of Al3+ in L1, L2 and L3 were analyzed. The results showed that L2 exhibited good sensitivity selectivity to Al3+. The fluorescence of L2 was strengthened dramatically in the presence of Al3+. Other metal ions, such as Cu2+, Hg2+, K+, Mg2+, Na+, Ni2+, Pb2+, Zn2+, Cr3+, Fe3+ induced negligible fluorescence strengthening for L2 under the same conditions. When the concentration (0~20 μmol/L) of Al3+ was increased, the fluorescence intensity of L2 (10 μmol/L) was also increased. The Job’s plot and fluorescence titration indicated that the stoichiometry of L2-Al3+ was 1:2 and the detection limit of Al3+ was 0.073 μmol/L. Moreover, L2 was used for the visual detection of Al3+.
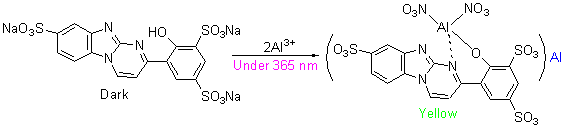
Ma Tuo , Zhang Jin , Liu Longzhu , He Yun , Zhang Zunting . Synthesis and Characterization of a Visualization and Water-Soluble Fluorescent Probe for Al3+[J]. Chinese Journal of Organic Chemistry, 2014 , 34(9) : 1780 -1785 . DOI: 10.6023/cjoc201405012
[1] Atkins, J.G.M.; Burgess, E.M.J.Am.Chem.Soc.1968, 17, 4744.
[2] Srinivasan, P.T.; Viraraghavan, T.; Subramanian, K.S.Water Sa.1999, 25, 47.
[3] Delhaize, E.; Ryan, P.R.J.Plant Physiol.1995, 107, 315.
[4] Godbold, D.L.; Fritz, E.; Hüttermann, A.Proc.Natl.Acad.Sci.U.S.A.1988, 11, 3888.
[5] Ren, J.; Tian, H.Sensors 2007, 12, 3166.
[6] Burwen, D.R.; Olsen, S.M.; Bland, L.A.; Arduino, M.J.; Reid, M.H.; Jarvis, W.R.Kidney.Int.1995, 2.
[7] Fasman, G.D.Coord.Chem.Rev.1996, 149, 125.
[8] Nayak, P.Environ.Res.2002, 89, 101.
[9] Cronan, C.S.; Walker, W.J.; Bloom, P.R.Nature 1986, 6093, 140.
[10] Ding, Y.; Li, X.; Li, T.; Zhu, W.-H.; Xie, Y.-S.J.Org.Chem.2013, 78, 5328.
[11] Li, P.; Zeng, Y.; Chen, J.P.; Li, Y.Y.; Li, Y.Acta Chim.Sinica 2012, 70, 1611 (in Chinese).(李鹏, 曾毅, 陈金平, 李迎迎, 李嫕, 化学学报, 2012, 70, 1611.)
[12] Berthon, G.Coord.Chem.Rev.2002, 228, 319
[13] Bielarczy, K.H.; Jankowska, A.; Madziar, B.; Matecki, A.; Michno, A.; Szutowicz, A.Neurochem.Int.2003, 42, 323.
[14] Yousef, M.I.; El-Morsy, A.; Hassan, M.S.Toxicology 2005, 215, 97.
[15] Sen, S.; Mukherjee, T.; Chattopadhyay, B.; Moirangthem, A.; Basu, A.; Marek, J.; Chattopadhyay, P.Analyst 2012, 17.
[16] Zhou, Y.; Zhang, J.; Zhou.; Zhang, L.; Zhang, M.Spectrochim.Acta, A 2013, 106, 68.
[17] Liu, L.Z.M.S.Thesis, Shaanxi Normal University, Xi'an 2013, p.41(刘龙珠, 硕士论文, 陕西师范大学, 西安, 2013, p.41.)
[18] Wang, L.N.; Qin, W.W.; Tang, X.L.; Dou, W.; Liu, W.; Teng, Q.; Yao, X.Org.Biomol.Chem.2010, 16, 3751.
/
| 〈 |
|
〉 |