

Chinese Journal of Organic Chemistry >
Highly Efficient Synthesis of 1,2,3-Triazoles Catalyzed by Silane Coupled Chitosan-CuBr Catalyst
Received date: 2014-04-14
Revised date: 2014-05-20
Online published: 2014-06-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21004024), the Natural Science Foundation of Fujian Province (No. 2011J01046), the Program for New Century Excellent Talents in Fujian Province (No. 2012FJ-NCET-ZR03) and the University Distinguished Young Research Talent Training Program of Fujian Province (No. 11FJPY02) and the Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (No. ZQN-YX103).
N-(2-Aminoethyl)-3-aminopropyltriethoxysilane-modified chitosan (CS-AAPTS) was successfully prepared by simply refluxing the corresponding CS and AAPTS in toluene solution. Subsequently, CS-AAPTS bound CuBr (CS-CuBr) was synthesized by the reaction of CS-AAPTS with CuBr in DMF at room temperature under N2 atmosphere. The obtained catalyst was characterized by FT-IR, TGA, XRD and EDX. The catalytic performances were evaluated in one-pot multicomponent copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction under microwave-assisted condition. CS-CuBr was found to exhibit obvious catalytic activity to rapidly prepare 1,2,3-triazole compounds under the microwave irradiation power of 480 W and 70 ℃ operating conditions. Furthermore, the catalyst could be easily recovered by simple filtration and recycled at least 4 cycles without significant loss of activity, and the preparation of 1,2,3-triazoles could be scaled up to multiple-gram conveniently with a yield up to 94%.
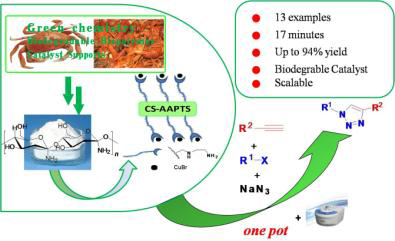
Key words: chitosan; heterogeneous catalyst; microwave-assisted; one-pot; 1,2,3-triazoles; CuAAC reaction
Jiang Yunbing , Wang Yanlong , Han Qian , Zhu Rongjun , Xiong Xingquan . Highly Efficient Synthesis of 1,2,3-Triazoles Catalyzed by Silane Coupled Chitosan-CuBr Catalyst[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 2068 -2075 . DOI: 10.6023/cjoc201404024
[1] Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem. Asian. J. 2011, 6, 2696.
[2] Bohacek, R. S.; McMartin, C.; Guida, W. C. Med. Res. Rev. 1996, 16, 3.
[3] Appukkuttan, P.; Dehaen, W.; Fokin, V. V.; Van der Eycken, E. Org. Lett. 2004, 6, 4223.
[4] Megia-Fernandez, A.; Ortega-Muñoz, M.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2012, 354, 1797.
[5] Driowya, M.; Puissant, A.; Robert, G.; Auberger, P.; Benhida, R.; Bougrin, K. Ultrason. Sonochem. 2012, 19, 1132.
[6] Xiong, X. Q.; Cai, L. Catal. Sci. Technol. 2013, 3, 1301.
[7] Kappe, C. O.; Van der Eycken, E. Chem. Soc. Rev. 2010, 39, 1280.
[8] Xiong, X. Q.; Cai, L.; Tang, Z. K. Chin. J. Org. Chem. 2012, 32, 1410 (in Chinese).
(熊兴泉, 蔡雷, 唐忠科, 有机化学, 2012, 32, 1410.)
[9] Lutz, J. F. Angew. Chem., Int. Ed. 2008, 47, 2182.
[10] Becer, C. R.; Hoogenboom, R.; Schubert, U. S. Angew. Chem., Int. Ed. 2009, 48, 4900.
[11] Dervaux, B.; Du Prez, F. E. Chem. Sci. 2012, 3, 959.
[12] Megia-Fernandez, A.; Ortega-Muñoz, M.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2010, 352, 3306.
[13] Hudson, R.; Li, C. J.; Moores, A. Green Chem. 2012, 14, 622.
[14] Baig, R. B. N.; Varma, R. S. Green Chem. 2012, 14, 625.
[15] Xiong, X. Q.; Chen, H. X.; Tang, Z. K.; Jiang, Y. B. RSC Adv. 2014, 4, 9830.
[16] Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Amiri Rudbari, H. J. Org. Chem. 2014, 79, 1437.
[17] Coelho, A.; Diz, P.; Caamano, O.; Sotelo, E. Adv. Synth. Catal. 2010, 352, 1179.
[18] Shamim, T.; Paul, S. Catal. Lett. 2010, 136, 260.
[19] Wan, L.; Cai, C. Catal. Lett. 2012, 142, 1134.
[20] Radatz, C. S.; do Amaral Soares, L.; Vieira, E. R.; Alves, D.; Russowsky, D.; Schneider, P. H. New J. Chem. 2014. 38, 1410.
[21] Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. J. Org. Chem. 2011, 76, 8394.
[22] Lopez Ruíz, H.; Emilio de la Cerda-Pedro, J.; Rojas-Lima, S.; Perez-Perez, I.; Viridiana Rodriguez-Sanchez, B.; Santillan, R.; Coreno, O. ARKIVOC 2013, (iii), 139.
[23] Lammens, M.; Skey, J.; Wallyn, S.; O'Reilly, R.; Du Prez, F. Chem. Commun. 2010, 46, 8719.
[24] Jalal, A.; Mosadegh, K.; Farhad, S.; Masoumeh, V. Catal. Commun. 2012, 27, 17.
[25] Roy, S.; Chatterjee, T.; Manirul Islam, S. Green Chem. 2013, 15, 2532.
[26] Xiong, X. Q.; Cai, L.; Jiang, Y. B.; Han, Q. ACS Sustainable Chem. Eng. 2014, 2, 765.
[27] Guibal, E. Prog. Polym. Sci. 2005, 30, 71.
[28] Baig, R. B. N.; Varma, R. S. Green Chem. 2013, 15, 1839.
[29] Wang, D.; Li, N.; Zhao, M. M.; Shi,W. L.; Ma, C. W.; Chen, B. H. Green Chem. 2010, 12, 2120.
[30] Doiron, J.; Soultan, A. H.; Richard, R.; Touré, M. M.; Picot, N.; Richard, R.; ?uperlovi?-Culf, M.; Robichaud, G. A.; Touaibia, M. Eur. J. Med. Chem. 2011, 46, 4010.
[31] Dilip, R. Catal. Commun. 2009, 10, 1240.
[32] Candelon, N.; Lastecoue res, D.; Diallo, A. K.; Aranzaes, J. R.; Astruc, D.; Vincent, J. M. Chem. Commun. 2008, 6, 741.
[33] Khan, S. S.; Hanelt, S., Liebscher, J. ARKIVOC 2009, (xii), 193.
[34] Iwasaki, M.; Yorimitsu, H.; Oshima, K. Chem. Asian J. 2007, 2, 1430.
/
| 〈 |
|
〉 |