

Chinese Journal of Organic Chemistry >
Recent Advances on Construction of Pyrrolophenanthridone Skeleton
Received date: 2014-04-22
Revised date: 2014-05-29
Online published: 2014-06-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272276) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1193).
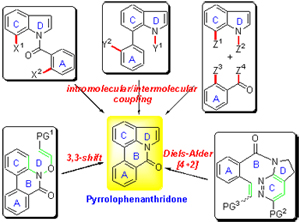
Pyrrolophenanthridone is a common structural motif existedexisting in a number of amaryllidaceae alkaloids, many of which possess a wide range of biological activities. The intriguing structural feature and significant biological activity have attracted much attention from synthetic communities. In the past decades, several excellent synthetic methodologies have been reported, in which intramolecular or intermolecular cross-coupling reaction through transition-metal catalyzed or radical pathway represents the most straightforward method for construction of this skeleton. This review focuses on the construction of pyrrolophenanthridone skeleton and total synthesis of amaryllidaceae alkaloids.
Cong Wei , Xu Jinyi , Yao Hequan . Recent Advances on Construction of Pyrrolophenanthridone Skeleton[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 1966 -1977 . DOI: 10.6023/cjoc201404041
[1] Martin, S. F. In The Alkaloids, Ed.: Brossi, A., Academic Press, New York, 1987, Vol. 30, Chapter 3, p. 251.
[2] (a) Pigni, N. B.; Ríos-Ruiz, S.; Martínez-Francés, V.; Nair, J. J.; Viladomat, F.; Codina, C.; Bastida, J. J. Nat. Prod. 2012, 75, 1643.
(b) Wang, L.; Zhang, X.-Q.; Yin, Z.-Q.; Wang, Y.; Ye, W.-C. Chem. Pharm. Bull. 2009, 57, 610.
(c) Ramadan, M. A.; Kamel, M. S.; Ohtani, K.; Kasai, R.; Yamasaki, K. Phytochemistry 2000, 54, 891.
(d) Ghosal, S.; Lochan, R.; Kumar, A. Y.; Srivastava, R. S. Phytochemistry 1985, 24, 1825.
(e) Ghosal, S.; Saini, K. S.; Frahm, A. W. Phytochemistry 1983, 22, 2305.
(f) Ghosal, S.; Rao, P. H.; Jaiswal, D. K.; Kumar, Y.; Frahm, A. W. Phytochemisrty 1981, 20, 2003.
[3] (a) Min, B. S.; Gao, J. J.; Nakamura, N.; Kim, Y. H.; Hattori, M. Chem. Pharm. Bull. 2001, 49, 1217.
(b) Ali, A. A.; Mesbah, M. K.; Frahm, A. W. Planta Med. 1981, 43, 407.
(c) Zee-Cheng, R. K.-Y.; Yan, S.-J.; Cheng, C. C. J. Med. Chem. 1978, 21, 199.
[4] Chattopadhyay, S.; Chattopadhyay, U.; Mathur, P. P.; Saini, K. S.; Ghosal, S. Planta Med. 1983, 49, 252.
[5] For selected reviews on transition-metal catalyzed functionalization of arenes, see:
(a) Johansson Seechurn, C. C. C.; Kitching, M. O.; Colacot, T. J.; Snieckus, V. Angew. Chem. Int. Ed. 2012, 51, 5062.
(b) Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236.
(c) Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936.
(d) Bras, J. L.; Muzart, J. Chem. Rev. 2011, 111, 1170.
(e) Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293.
(f) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(g) Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20.
(h) Johansson, C. C. C.; Colacot, T. J. Angew. Chem., Int. Ed. 2010, 49, 676.
(i) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
[6] For a selected review on palladium-catalyzed intramolecular cylization of heteroarenes, see:
(a) You, S.-L.; Xia, J.-B. Top. Curr. Chem. 2010, 292, 165.
For selected examples, see:
(b) Zhao, L.; Li, Z.; Chang, L.; Xu, J.; Yao, H.; Wu, X. Org. Lett. 2012, 14, 2066.
(c) Piou, T.; Neuville, L.; Zhu, J. Tetrahedron 2013, 69, 4415.
(d) Wu, K.-J.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 3772.
(e) Lv, J.; Liu, Q.; Tang, J.; Perdih, F.; Kranjc, K. Tetrahedron Lett. 2012, 53, 5248.
(f) Yamuna, E.; Zeller, M.; Prasad, K. J. R. Tetrahedron Lett. 2011, 52, 6030.
(g) Wang, C.; Piel, I.; Glorius, F. J. Am. Chem. Soc. 2009, 131, 4194.
(h) Rudolph, A.; Rackelmann, N.; Turcotte-Savard, M.-O.; Lautens, M. J. Org. Chem. 2009, 74, 289.
(i) Zhang, H.; Ferreira, E. M.; Stoltz, B. M. Angew. Chem., Int. Ed. 2004, 43, 6144.
(j) Huang, Q.; Fazio, A.; Dai, G.; Campo, M. A.; Larock, R. C. J. Am. Chem. Soc. 2004, 126, 7460.
(k) Ferreira, E. M.; Stoltz, B. M. J. Am. Chem. Soc. 2003, 125, 9578.
(l) Campo, M. A.; Huang, Q.; Yao, T.; Tian, Q.; Larock, R. C. J. Am. Chem. Soc. 2003, 125, 11506.
[7] Grigg, R.; Teasdale, A.; Sridharan, V. Tetrahedron Lett. 1991, 32, 3859.
[8] Sakamoto, T.; Yasuhara, A.; Kondo, Y.; Yamanaka, H. Heterocycles 1993, 36, 2597.
[9] Knölker, H.-J.; Filali, S. Synlett 2003, 1752.
[10] For selected examples, see:
(a) Pinto, A. C.; Silva, F. S. Q.; Silva, R. B. Tetrahedron Lett. 1994, 35, 8923.
(b) Garden, S. J.; Silva, R. B.; Pinto, A. C. Tetrahedron 2002, 58, 8399.
[11] Torres, J. C.; Pinto, A. C.; Garden, S. J. Tetrahedron 2004, 60, 9889.
[12] Miki, Y.; Shirokoshi, H.; Matsushita, K. Tetrahedron Lett. 1999, 40, 4347.
[13] Umemoto, H.; Dohshita, M.; Hamamoto, H.; Miki, Y. Heterocycles 2011, 83, 1111.
[14] Miki, Y.; Umemoto, H.; Dohshita, M.; Hamamoto, H. Tetrahedron Lett. 2012, 53, 1924.
[15] Cong, W.; Zhao, L.; Wu, X.; Xu, J.; Yao, H. Tetrahedron 2014, 70, 312.
[16] For selected examples, see:
(a) Xie, Y.; Yang, Y.; Huang, L.; Zhang, X.; Zhang, Y. Org. Lett. 2012, 14, 1238.
(b) Wang, Z.-Q.; Zhang, W.-W.; Gong, L.-B.; Tang, R.-Y.; Yang, X.-H.; Liu, Y.; Li, J.-H. Angew. Chem., Int. Ed. 2011, 50, 8968.
[17] Rosen, B. M.; Percec, V. Chem. Rev. 2009, 109, 5069.
[18] Lauk, U.; Dürst, D.; Fischer, W. Tetrahedron Lett. 1991, 32, 65.
[19] Tsuge, O.; Hatta, T.; Tsuchiyama, H. Chem. Lett. 1998, 155.
[20] Sperotto, E.; van Klink, G. P.; van Koten, G.; de Vries, J. G. Dalton Trans. 2010, 39, 10338.
[21] Ziegler, F. E.; Chliwner, I.; Fowler, K. W.; Kanfer, S. J.; Kuo, S. J.; Sinha, N. D. J. Am. Chem. Soc. 1980, 102, 790.
[22] Harrowven, D. C.; Lai, D.; Lucas, M. C. Synthesis 1999, 1300.
[23] Hiroya, K.; Itoh, S.; Sakamoto, T. J. Org. Chem. 2004, 69, 1126.
[24] Ganton, M. D.; Kerr, M. A. Org. Lett. 2005, 7, 4777.
[25] Iwao, M.; Takehara, H.; Obta, S.; Watanabe, M. Heterocycles 1994, 38, 1717.
[26] Harutyunyan, S. R.; den Hartog, T.; Geurts, K.; Minnaard, A. J.; Feringa, B. L. Chem. Rev. 2008, 108, 2824.
[27] Hutchings, R. H.; Meyers, A. I. J. Org. Chem. 1996, 61, 1004.
[28] For selected examples, see:
(a) Fodor, G.; Nagubandi, S. Tetrahedron 1980, 36, 1279.
(b) Medley, J. W.; Movassaghi, M. Org. Lett. 2013, 15, 3614.
(c) Su, B.; Chen, F.; Wang, Q. J. Org. Chem. 2013, 78, 2775.
(d) Adachi, S.; Onozuka, M.; Yoshida, Y.; Ide, M.; Saikawa, Y.; Nakata, M. Org. Lett. 2014, 16, 358.
[29] Banwell, M. G.; Bissett, B. D.; Busato, S.; Cowden, C. J.; Hockless, D. C. R.; Holman, J. W.; Read, R. W.; Wu, A. W. J. Chem. Soc., Chem. Commun. 1995, 2551.
[30] Hiroya, K.; Itoh, S.; Sakamoto, T. J. Org. Chem. 2004, 69, 1126.
[31] Siddiqui, M. A.; Snieckus, V. Tetrahedron Lett. 1990, 31, 1523.
[32] Hartung, C. G.; Fecher, A.; Chapell, B.; Snieckus, V. Org. Lett. 2003, 5, 1899.
[33] Mentzel, U. V.; Tanner, D.; Tønder, J. E. J. Org. Chem. 2006, 71, 5807.
[34] Robbins, D. W.; Boebel, T. A.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 4068.
[35] Kim, H. S.; Banwell, M. G.; Willis, A. C. J. Org. Chem. 2013, 78, 5103.
[36] Pereira, M. M. A.; Prabhakar, S.; Lobo, A. M. J. Nat. Prod. 1996, 59, 744.
[37] For selected examples, see:
(a) Jia, Z.-J.; Jiang, H.; Li, J.-L.; Gschwend, B.; Li, Q.-Z.; Yin, X.; Grouleff, J.; Chen, Y.-C.; Jørgensen, K. A. J. Am. Chem. Soc. 2011, 133, 5053.
(b) Liu, Y.; Nappi, M.; Arceo, E.; Vera, S.; Melchiorre, P. J. Am. Chem. Soc. 2011, 133, 15212.
(c) Lin, S.; Ischay, M. A.; Fry, C. G.; Yoon, T. P. J. Am. Chem. Soc. 2011, 133, 19350. For selected reviews, see:
(d) Jiang, X.; Wang, R. Chem. Rev. 2013, 113, 5515.
(e) Funel, J. A.; Abele, S. Angew. Chem., Int. Ed. 2013, 52, 3822.
[38] Boger, D. L.; Wolkenberg, S. E. J. Org. Chem. 2000, 65, 9120.
/
| 〈 |
|
〉 |