

Chinese Journal of Organic Chemistry >
Synthesis of α-Siloxyamides by the Reaction of a Carbamoylsilane with Ketones
Received date: 2014-04-25
Revised date: 2014-05-21
Online published: 2014-06-16
Supported by
Project supported by the Foundation for Returness Overseas Scientists of Shanxi Province (No. 0713), the Natural Science Foundation of Shanxi Province (No. 2012011046-9), and the Foundation of Shanxi Normal University (No. SD2012CXSY-11).
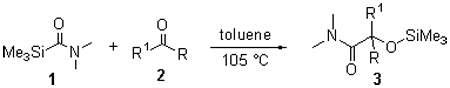
Under using anhydrous toluene as a solvent, N,N-dimethyl carbamoyl(trimethyl)silane reacted with a series of aryl ketones or unsaturated aryl ketones at 105 ℃ to afford α-siloxyamide derivatives in good yields (60%~89%). The structures of the products were characterized by element analysis, 1H NMR, 13C NMR and IR spectra. The alkyl ketones including the alkyl-aryl ketones and alkyl-unsaturated ketones poorly reacted with N,N-dimethylcarbamoyl(trimethyl)silane. The experimental results showed that the electronic property of the substituted group on the aryl ring strongly affected the reaction. In the case of the aryl ketones with stronger donor group on the aryl ring, the longer time was needed for the reaction.
Key words: carbamoylsilane; ketones; α-siloxyamides; nucleophilic addition; synthetic methods
Yao Yuan , Li Weidong , Chen Jianxin . Synthesis of α-Siloxyamides by the Reaction of a Carbamoylsilane with Ketones[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 2124 -2129 . DOI: 10.6023/cjoc201404048
[1] Babine, R. E.; Bender, S. L. Chem. Rev. 1997, 97, 1359.
[2] Sakurai, M.; Higashida, S.; Sugano, M.; Komai, T.; Yagi, R.; Ozawa, Y.; Handa, H.; Nishigaki, T.; Yabe, Y. Bioorg. Med. Chem. 1994, 2, 807.
[3] Soeta, T.; Kojima, Y.; Ukaji, Y.; Inomata, K. Tetrahedron Lett. 2011, 52, 2557.
[4] Kumar, J. S.; Jonnalagadd, S. C.; Mereddy, V. R. Tetrahedron Lett. 2010, 51, 779.
[5] Bowen, S. M.; Duesler, E. N.; Paine, R. T. Inorg. Chem. 1982, 21, 261.
[6] Kuduk, S. D.; Chang, R. K.; Dipardo, R. M.; Di Marco, C. N.; Murphy, K. L.; Ransom, R. W.; Reiss, D. R.; Tang, C.; Prueksari- tanont, T.; Pettibone, D. J.; Boch, M. G. Bioorg. Med. Chem. Lett. 2008, 18, 5107
[7] Schollkopf, U.; Beckhaus, H. Angew. Chem., Int. Ed. Engl. 1976, 15, 293.
[8] Ramon, D. J.; Yus, M. Tetrahedron Lett. 1993, 34, 7115.
[9] Nudelman, N. S.; Linares, G. E. G. J. Org. Chem. 2000, 65, 1629.
[10] Banfi, L.; Riva, R. Org. React. 2005, 65, 1.
[11] Domling, A.; Ugi, L. Angew. Chem., Int. Ed. 2000, 39, 3168.
[12] Yue, T.; Wang, M.-X.; Wang, D.-X.; Masson, G.; Zhu, J. Angew. Chem., Int. Ed. 2009, 48, 6717.
[13] Mihara, H.; Xu, Y.; Shepherd, N. E.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 8384.
[14] Chen, J.-X.; Cunico, R. F. Tetrahedron Lett. 2002, 43, 8595.
[15] Chen, J.-X.; Cunico, R. F. Tetrahedron Lett. 2003, 44, 8025.
[16] Chen, J.-X.; Pandey, R. K.; Cunico, R. F. Tetrahedron: Asymmmetry 2005, 16, 941.
[17] Chen, J.-X.; Wen, X.-S. Acta Chim. Sinica 2009, 64, 1709 (in Chinese).
(陈建新, 温雪山, 化学学报, 2009, 64, 1709.)
[18] Chen, J.-X.; Cunico, R. F. J. Org. Chem. 2004, 69, 5509.
[19] Chen X.-J.; Chen, J.-X. Mendeleev Commun. 2013, 23, 106.
[20] Ma, F.; Chen, J.-X. Acta Chim. Sinica 2013, 71, 1118 (in Chinese).
(马菲, 陈建新, 化学学报, 2013, 71, 1118.)
[21] Cunico, R. F. Tetrahedron Lett. 2002, 43, 355.
[22] Carbamoylsilane were prepared as reported, see: Cunico, R. F.; Chen, J.-X. Synth. Commun. 2003, 33, 1963.
/
| 〈 |
|
〉 |