

Chinese Journal of Organic Chemistry >
Synthesis of Cyclopalladated Phenyl Imidazoline-Carbene Complexes and Catalytic Study in Suzuki Reaction
Received date: 2014-05-17
Revised date: 2014-06-16
Online published: 2014-07-02
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21102135, 21272217, J1210060).
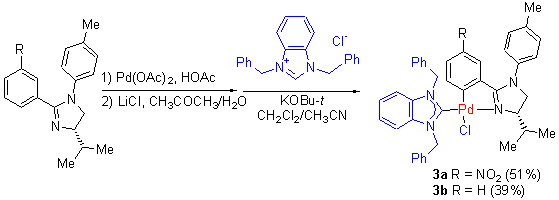
The amido alcohols compounds 1a and 1b were synthesized from commercially available 3-nitrobenzoic acid or benzoic acid and (S)-2-amino-3-methyl-1-butanol at room temperature. The amido alcohols, when treated with excess thionyl chloride, followed by p-toluidine, gave the phenyl imidazoline compounds 2a and 2b. Cyclopalladated phenyl imidazoline-carbene complexes 3a and 3b were obtained by the reaction of phenyl imidazolines 2a and 2b with Pd(OAc)2/LiCl followed by treatment with 1,3-dibenzyl-benzimidazolium chloride. All the complexes 3a and 3b were characterized by 1H NMR, 13C NMR, MS and elemental analysis. In the crystal of complex 3a, the compound was assembled to a chain supramolecular structure by the intermolecular hydrogen bonds. And complex 3a presented good catalytic effect on Suzuki reaction.
Key words: cyclopalladated; phenyl imidazoline; carbene complex; crystal structure; Suzuki
Zhao Xuemei , Xiao Zhiqiang , Liang Ting , Gao Xiang , Hao Xinqi , Song Maoping . Synthesis of Cyclopalladated Phenyl Imidazoline-Carbene Complexes and Catalytic Study in Suzuki Reaction[J]. Chinese Journal of Organic Chemistry, 2014 , 34(11) : 2304 -2308 . DOI: 10.6023/cjoc201405026
[1] (a) Zapf, A. Transition Metals for Organic Synthesis, Vol. 1, 2nd ed., Eds: Beller, M.; Bolm, C., Wiley-VCH, Weinheim, 2004, p. 211. (b) Miyaura, N. Metal-Catalyzed Cross-Coupling Reactions, Vol. 1, 2nd ed., Eds: Meijere, A. de; Diederich, F., Wiley-VCH, Weinheim, 2004, p. 41.
[2] (a) Littke, A. F.; Dai, C.; Fu, G. C. J. Am. Chem. Soc. 2000, 122, 4020. (b) Pickett, T. E.; Roca, F. X.; Richards, C. J. J. Org. Chem. 2003, 68, 2592. (c) Weng, Z.; Teo, S.; Koh, L. L.; Hor, T. S. A. Organometallics 2004, 23, 4342. (d) Walker, S. D.; Barder, T. E.; Martinelli, J. R.; Buchwald, S. L. Angew. Chem., Int. Ed. 2004, 43, 1871. (e) Kwong, F. Y.; Lam, W. H.; Yeung, C. H.; Chan, K. S.; Chan, A. S. C. Chem. Commun. 2004, 1922.
[3] (a) Viciu, M. S.; Kelly III, R. A.; Stevens, E. D.; Naud, F.; Studer, M.; Nolan, S. P. Org. Lett. 2003, 5, 1479. (b) Lewis, A. K. de K.; Caddick, S.; Cloke, F. G. N.; Billingham, N. C.; Hitchcock, P. B.; Leonard, J. J. Am. Chem. Soc. 2003, 125, 10066.(c) Frey, G. D.; Schütz, J.; Herdtweck, E.; Herrmann, W. A. Organometallics 2005, 24, 4416. (d) Peris, E.; Crabtree, R. H. Coord. Chem. Rev. 2004, 248, 2239.
[4] (a) Navarro, O.; Kelly III, R. A.; Nolan, S. P. J. Am. Chem. Soc. 2003, 125, 16194. (b) Navarro, O.; Marion, N.; Oonishi, Y.; Kelly III, R. A.; Nolan, S. P. J. Org. Chem. 2006, 71, 685.
[5] Frey, G. D.; Schütz, J.; Herrmann, W. A. J. Organomet. Chem. 2006, 691, 2403.
[6] (a) Iyer, S.; Jayanthi, A. Synlett 2003, 1125. (b) Iyer, S.; Kulkarni, G. M.; Ramesh, C. Tetrahedron 2004, 60, 2163.
[7] Bedford, R. B.; Betham, M.; Blake, M. E.; Frost, R. M.; Horton, P. N.; Hursthouse, M. B.; López-Nicolás, R.-M. Dalton Trans. 2005, 2774.
[8] Shi, J.-C.; Cao, X.-H.; Zheng, Y.; Jia, L. Chin. J. Org. Chem. 2007, 27, 666 (in Chinese).(施继成, 曹新华, 郑瑛, 贾莉, 有机化学, 2007, 27, 666.)
[9] Jiang, L.; Li, Z. N.; Zhao, D. F. Chin. J. Org. Chem. 2010, 30, 200 (in Chinese).(姜岚, 李争宁, 赵德峰, 有机化学, 2010, 30, 200.)
[10] (a) Li, J.-Y.; Wu, Y.-J.; Han, Z.-X.; Jiang, S. Chem. J. Chin. Univ. 2008, 29, 1555 (in Chinese).(李敬亚, 吴养洁, 韩自省, 姜松, 高等学校化学学报, 2008, 29, 1555). (b) Li, J.-Y.; Yu, A-J.; Wu, Y.-J.; Zhu, Y.; Du, C.-X.; Yang, H.-W. Polyhedron 2007, 26, 2629.
[11] Sekar, G.; DattaGupta, A.; Singh, V. K. J. Org. Chem. 1998, 63, 2961.
[12] Boland, N. A.; Casey, M.; Hynes, S. J.; Matthews, J. W.; Smyth, M. P. J. Org. Chem. 2002, 67, 3919.
[13] McKennon, M. J.; Meyers, A. I.; Drauz, K.; Schwarm, M. J. Org. Chem. 1993, 58, 3568.
[14] Huang, W.; Guo, J. P.; Xiao, Y. J.; Zhu, M. F.; Zou, G.; Tang, J. Tetrahedron 2005, 61, 9783.
[15] Sheldrick, G. M. SHELXL-97, Program for Crystal Structures Refinement, University of Göttingen, Germany, 1997.
/
| 〈 |
|
〉 |