

Chinese Journal of Organic Chemistry >
Research on Particle Size of Organic Semiconductor Materials Poly(3-hexylthiophene) and [6,6]-Phenyl-C60-butyric Acid Methyl Ester in Chlorobenzene Solution
Received date: 2014-05-20
Revised date: 2014-06-23
Online published: 2014-07-03
Supported by
Project supported by the National Natural Science Foundation of China (No.11074066).
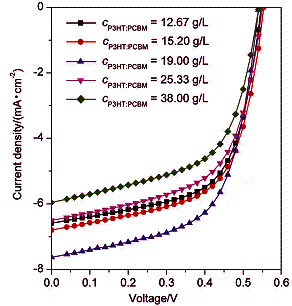
The mixture of organic semiconductor electron donor poly(3-hexylthiophene) (P3HT) and acceptor [6,6]-phenyl- C60-butyric acid methyl ester (PCBM) is dissolved in chlorobenzene for measuring the solute particle diameter. By comparing the performance of solar cells and the surface morphology of the active layer, the dispersion law of the mixed solutes in chlorobenzene is analyzed. The influence of temperature and concentration on particle size, furthermore, the impact of particle size on device performance are discussed. The results show that most of the particle sizes in the solution are populated at about 4000 nm, and both the concentration and the temperature of the solution have a significant effect on the particle size of the solution. For low concentration solution, the particle sizes are greatly influenced by temperature. On the contrary, the effect of temperature on the particle size in the solution concentration is less apparent when the concentration becomes higher. When the solution concentration reaches 12.67 mg/mL, it has a preferably dispersion and optimal fill factor. When the concentration reaches 19.00 mg/mL, the solar cell made by the solution shows a well property on power conversion efficiency and short-circuits current.
Key words: polymer; chlorobenzene; particle size; organic solar cells; P3HT:PCBM
Li Chenxi , Dong Bingchao , Li Meng , Wang Jingmiao , Cai Wenjun , Niu Heying , Ma Heng . Research on Particle Size of Organic Semiconductor Materials Poly(3-hexylthiophene) and [6,6]-Phenyl-C60-butyric Acid Methyl Ester in Chlorobenzene Solution[J]. Chinese Journal of Organic Chemistry, 2014 , 34(11) : 2370 -2375 . DOI: 10.6023/cjoc201405029
[1] Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. Nat. Mater. 2005, 4, 864.
[2] Koster, L.; Mihailetchi, V. D.; Blom, P. Appl. Phys. Lett. 2006, 88, 93511.
[3] Hauch, J. A.; Schilinsky, P.; Choulis, S. A.; Childers, R.; Biele, M.; Brabec, C. J. Sol. Energy Mater. Sol. Cells 2008, 92, 727.
[4] Tsoi, W. C.; Spencer, S. J.; Yang, L.; Ballantyne, A. M.; Nicholson, P. G.; Turnbull, A.; Shard, A. G.; Murphy, C. E.; Bradley, D. D.; Nelson, J. Macromolecules 2011, 44, 2944.
[5] Coakley, K. M.; Mcgehee, M. D. Chem. Mater. 2004, 16, 4533.
[6] Chang L.; Lademann, H. A.; Bonekamp, J. B.; Meerholz, K.; Moulé, A. J. Adv. Funct. Mater. 2011, 21, 1779.
[7] Janssen, G.; Aguirre, A.; Goovaerts, E.; Vanlaeke, P.; Poortmans, J.; Manca, J. Eur. Phys. J. Appl. Phys. 2007, 37, 287.
[8] He P.; Li Z. F.; Hou Q. F.; Wang Y. L. Chin. J. Org. Chem. 2013, 33, 288 (in Chinese).(和平, 李在房, 侯秋飞, 王艳玲, 有机化学, 2013, 33, 288.)
[9] Lee, J. M.; Park, J. S.; Lee, S. H.; Kim, H.; Yoo, S.; Kim, S. O. Adv. Mater. 2011, 23, 629.
[10] Zhao, G.; He, Y.; Xu, Z.; Hou, J.; Zhang, M.; Min, J.; Chen, H.; Ye, M.; Hong, Z.; Yang, Y. Adv. Funct. Mater. 2010, 20, 1480.
[11] Wei, G. M.; Yao, D. D.; Li, Z. Y.; Huang, Y.; Cheng, H.; Han, C. C. Macromolecules 2013, 46, 1212.
[12] Thompson, B. C.; Fréchet, J. M. Angew. Chem., Int. Ed. 2008, 47, 58.
[13] Zhou, E.; Nakamura, M.; Nishizawa, T.; Zhang, Y.; Wei, Q.; Tajima, K.; Yang, C.; Hashimoto, K. Macromolecules 2008, 41, 8302.
[14] Mihailetchi, V. D.; Xie, H. X.; De boer, B.; Koster, L. A.; Blom, P. W. Adv. Funct. Mater. 2006, 16, 699.
[15] Song, X.; Hua, W.; Ma, Y.; Wang, C.; Luo, Y. J. Phys. Chem. C 2012, 116, 22394.
[16] Hoppe, H.; Sariciftci, N. S. J. Mater. Chem. 2006, 16, 45.
[17] Chen, D.; Nakahara, A.; Wei, D.; Nordlund, D.; Russell, T. P. Nano Lett. 2010, 11, 561.
[18] Huang, Y; Cheng, H; Han, C. C. Macromolecules 2011, 44, 5020.
[19] Alibrahim, M.; Ambacher, O.; Sensfuss, S.; Gobsch, G. Appl. Phys. Lett. 2005, 86, 201120.
[20] Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A. J. Adv. Funct. Mater. 2005, 15, 1617.
[21] Tenhulscher, T. E.; Vandervelde, L. E.; Bruggeman, W. A. Environ. Toxicol. Chem. 1992, 11, 1595.
[22] Campoy-quiles, M.; Ferenczi, T.; Agostinelli, T.; Etchegoin, P. G.; Kim, Y.; Anthopoulos, T. D.; Stavrinou, P. N.; Bradley, D. D.; Nelson, J. Nat. Mater. 2008, 7, 158.
[23] Liu, Z.; Xu, F.; Yan, D. D. Acta Chim. Sinica 2014, 72, 171 (in Chinese).(刘震, 徐丰, 严大东, 化学学报, 2014, 72, 171.)
[24] Vanlaeke, P.; Swinnen, A.; Haeldermans, I.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; D'haen, J.; Heremans, P.; Poortmans, J. Sol. Energy Mater. Sol. Cells 2006, 90, 2150.
/
| 〈 |
|
〉 |