

Chinese Journal of Organic Chemistry >
Progress in Phosphine-Promoted Annulations between Two Electrophiles
Received date: 2014-06-29
Revised date: 2014-08-12
Online published: 2014-08-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272119), the Qualified Personnel Foundation of Taiyuan University of Technology (No. tyutrc-201357a), and the Youth Foundation of Taiyuan University of Technology (No. 2013Z043).
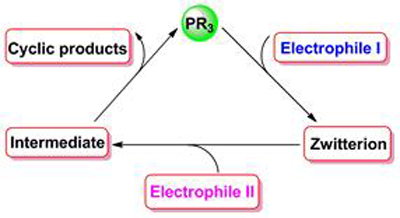
The development of highly efficient synthetic methods of cyclic compounds is of great significance in the syntheses of pharmaceutically active molecules, natural products and other functional organic molecules. Recently, phosphine-promoted annulations of two electrophiles, which provide highly efficient access to various carbo-and heterocycles, have attracted extensive interest from synthetic chemists due to their merits such as ready availability of starting materials, mild and metal-free conditions. Generally, this kind of annulation reaction proceeds through a key step to generate an active zwitterionic intermediate via nucleophilic addition of the phosphine to an electrophile. According to different sources of the zwitterions, this review summarizes the recent progress in phosphine-promoted annulations of electron-deficient allenes, Morita-Baylis-Hillman allylic adducts, and electron-deficient alkenes with other electrophiles.
Key words: phosphine; electrophile; zwitterions; annulations
Zhou Rong , Liu Rongfang , Li Ruifeng , He Zhengjie . Progress in Phosphine-Promoted Annulations between Two Electrophiles[J]. Chinese Journal of Organic Chemistry, 2014 , 34(12) : 2385 -2405 . DOI: 10.6023/cjoc201406049
[1] Quin, L.D. A Guide to Organophosphorus Chemistry, Wiley, New York, 2000.
[2] (a) Wittig, G.; Schöllkopf, U. Chem. Ber. 1954, 87, 1318. (b) Mitsunobu, O. Synthesis 1981, 1. (c) Staudinger, H.; Meyer, J. Helv. Chim. Acta 1919, 2, 635. (d) Xu, S.; He, Z. Chin. J. Org. Chem. 2012, 32, 1159 (in Chinese). (徐四龙, 贺峥杰, 有机化学, 2012, 32, 1159.)
[3] (a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535. (b) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 348, 1035. (c) Tang, Q.; Tu, A.; Deng, Z.; Hu, M.; Zhong, W. Chin. J. Org. Chem. 2013, 33, 954 (in Chinese). (唐谦, 涂爱平, 邓真真, 胡梦莹, 钟为慧, 有机化学, 2013, 33, 954.) (d) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140. (e) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102. (f) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724. (g) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588. (h) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927.
[4] Zhang, C.; Lu, X. J. Org. Chem. 1995, 60, 2906.
[5] (a) Xia, Y.; Liang, Y.; Chen, Y.; Wang, M.; Jiao, L.; Huang, F.; Liu, S.; Li, Y.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 3470. (b) Liang, Y.; Liu, S.; Xia, Y.; Li, Y.; Yu, Z.-X. Chem.-Eur. J. 2008, 14, 4361.
[6] (a) Mercier, E.; Fonovic, B.; Henry, C.; Kwon, O.; Dudding, T. Tetrahedron Lett. 2007, 48, 3617. (b) Dudding, T.; Kwon, O.; Mercier, E. Org. Lett. 2006, 8, 3643.
[7] (a) Xu, Z.; Lu, X. J. Org. Chem. 1998, 63, 5031. (b) Xu, Z.; Lu, X. Tetrahedron Lett. 1999, 40, 549. (c) Xu, Z.; Lu, X. Tetrahedron Lett. 1997, 38, 3461.
[8] Zhu, X.-F.; Henry, C. E.; Kwon, O. Tetrahedron 2005, 61, 6276.
[9] Zhang, B.; He, Z.; Xu, S.; Wu, G.; He, Z. Tetrahedron 2008, 64, 9471.
[10] (a) Hartley, R. C.; Caldwell, S. T. J. Chem. Soc., Perkin Trans. 1 2000, 477. (b) Yong, S. R.; Williams, M. C.; Pyne, S. G.; Ung, A. T.; Skelton, B. W.; White, A. H.; Turner, P. Tetrahedron 2005, 61, 8120.
[11] (a) Pham, T. Q.; Pyne, S. G.; Skelton, B. W.; White, A. H. J. Org. Chem. 2005, 70, 6369. (b) Wu, H.; Zhang, H.; Zhao, G. Tetrahedron 2007, 63, 6454. (c) Du, Y.; Lu, X. J. Org. Chem. 2003, 68, 6463. (d) Wang, J.-C.; Krische, M. J. Angew. Chem., Int. Ed. 2003, 42, 5855. (e) Andrews, I. P.; Kwon, O. Chem. Sci. 2012, 3, 2510.
[12] Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Chem. Eur. J. 2009, 15, 8698.
[13] Wang, T.; Ye, S. Org. Biomol. Chem. 2011, 9, 5260.
[14] Zhu, X.-F.; Lan, J.; Kwon, O. J. Am. Chem. Soc. 2003, 125, 4716.
[15] (a) Tran, Y. S.; Kwon, O. J. Am. Chem. Soc. 2007, 129, 12632. (b) Tran, Y. S.; Martin, T. J.; Kwon, O. Chem. Asian J. 2011, 6, 2101.
[16] (a) Villa, R. A.; Xu, Q.; Kwon, O. Org. Lett. 2012, 14, 4634. (b) Tran, Y. S.; Kwon, O. Org. Lett. 2005, 7, 4289.
[17] (a) Wurz, R. P.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 12234. (b) Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Chem. Sci. 2012, 3, 1231. (c) Xiao, H.; Chai, Z.; Cao, D.; Wang, H.; Chen, J.; Zhao, G. Org. Biomol. Chem. 2012, 10, 3195.
[18] Wang, T.; Ye, S. Org. Lett. 2010, 12, 4168.
[19] Li, E.; Huang, Y.; Liang, L.; Xie, P. Org. Lett. 2013, 15, 3138.
[20] Gicquel, M.; Gomez, C.; Retailleau, P.; Voituriez, A.; Marinetti, A. Org. Lett. 2013, 15, 4002.
[21] (a) Meng, X. T.; Huang, Y.; Chen, R. Y. Org. Lett. 2009, 11, 137. (b) Sun, Y.-W.; Guan, X.-Y.; Shi, M. Org. Lett. 2010, 12, 5664. (c) Kumar, K.; Kapoor, R.; Kapur, A.; Ishar, M. P. S. Org. Lett. 2000, 2, 2023. (d) Kumar, K.; Kapur, A.; Ishar, M. P. S. Org. Lett. 2000, 2, 787. (e) Liu, B.; Davis, R.; Joshi, B.; Reynolds, D. W. J. Org. Chem. 2002, 67, 4595. (f) Zhu, X. F.; Henry, C. E.; Wang, J.; Dudding, T.; Kwon, O. Org. Lett. 2005, 7, 1387. (g) Zhu, X.-F.; Schaffner, A.-P.; Li, R. C.; Kwon, O. Org. Lett. 2005, 7, 2977. (h) Creech, G. S.; Kwon, O. Org. Lett. 2008, 10, 429. (i) Jing, C,; Na, R.; Wang, B.; Liu, H.; Zhang, L.; Liu, J.; Wang, M.; Zhong, J.; Kwon, O.; Guo, H. Adv. Synth. Catal. 2012, 354, 1023. (j) Zheng, J.; Huang, Y.; Li, Z. Org. Lett. 2013, 15, 5064. (k) Li, E.; Jia, P.; Liang, L.; Huang, Y. ACS Catal. 2014, 4, 600. (l) Li, E.; Huang, Y. Chem. Commun. 2014, 50, 948. (m) Li, E.; Huang, Y. Chem. Eur. J. 2014, 20, 3520.
[22] Guo, H.; Xu, Q.; Kwon, O. J. Am. Chem. Soc. 2009, 131, 6318.
[23] Na, R.; Jing, C.; Xu, Q.; Jiang, H.; Wu, X.; Shi, J.; Zhong, J.; Wang, M.; Benitez, D.; Tkatchouk, E.; Goddard, W. A.; Guo, H.; Kwon, O. J. Am. Chem. Soc. 2011, 133, 13337.
[24] Du, Y.; Lu, X.; Zhang, C. Angew. Chem., Int. Ed. 2003, 42, 1035.
[25] Feng, J.; Lu, X.; Kong, A.; Han, X. Tetrahedron 2007, 63, 6035.
[26] Zheng, S.; Lu, X. Tetrahedron Lett. 2009, 50, 4532.
[27] Deng, H.-P.; Wei, Y.; Shi, M. Org. Lett. 2011, 13, 3348.
[28] Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Angew. Chem., Int. Ed. 2011, 50, 7837.
[29] Tan, B.; Candeias, N. R.; Barbas, C. F. J. Am. Chem. Soc. 2011, 133, 4672.
[30] Zhong, F.; Chen, G.-Y.; Han, X.; Yao, W.; Lu, Y. Org. Lett. 2012, 14, 3764.
[31] Zheng, S.; Lu, X. Org. Lett. 2008, 10, 4481.
[32] Du, Y.; Feng, J.; Lu, X. Org. Lett. 2005, 7, 1987.
[33] Zheng, S.; Lu, X. Org. Lett. 2009, 11, 3978.
[34] Zhou, R.; Wang, J.; Song, H.; He, Z. Org. Lett. 2011, 13, 580.
[35] Chen, Z.; Zhang, J. Chem. Asian J. 2010, 5, 1542.
[36] Tian, J.; Zhou, R.; Sun, H.; Song, H.; He, Z. J. Org. Chem. 2011, 76, 2374.
[37] Zhou, R.; Duan, C.; Yang, C.; He, Z. Chem. Asian. J. 2014, 9, 1183.
[38] Xie, P.; Huang, Y.; Chen, R. Org. Lett. 2010, 12, 3768.
[39] (a) Zhang, X.; Deng, H.-P.; Huang, L.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 8664. (b) Hu, F.-L.; Wei, Y.; Shi, M. Chem. Commun. 2014, 50, 8912.
[40] Xie, P.; Huang, Y.; Chen, R. Chem. Eur. J. 2012, 18, 7362.
[41] Xie, P.; Li, E.; Zheng, J.; Li, X.; Huang, Y.; Chen, R. Adv. Synth. Catal. 2013, 355, 161.
[42] Zheng, J.; Huang, Y.; Li, Z. Chem. Commun. 2014, 50, 5710.
[43] Aroyan, C. E.; Dermenci, A.; Miller, S. J. Tetrahedron 2009, 65, 4069.
[44] (a) Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811. (b) Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447. (c) Cui, P.-L.; Wang, C.; Guo, X.-M.; Liu, H.-Y.; Feng, T. Chin. J. Org. Chem. 2008, 28, 194 (in Chinese). (崔朋雷, 王春, 果秀敏, 刘海燕, 冯涛, 有机化学, 2008, 28, 194.)
[45] Cai, L.; Zhang, B.; Wu, G.; Song, H.; He, Z. Chem. Commun. 2011, 47, 1045.
[46] Zhou, R.; Wang, J.; Tian, J.; He, Z. Org. Biomol. Chem. 2012, 10, 773.
[47] Hu, F.-L.; Wei, Y.; Shi, M. Adv. Synth. Catal. 2014, 356, 736.
[48] Zhou, R.; Wang, J.; Yu, J.; He, Z. J. Org. Chem. 2013, 78, 10596.
[49] Ma, J.; Xie, P.; Hu, C.; Huang, Y.; Chen, R. Chem. Eur. J. 2011, 17, 7418.
[50] Schuler, M.; Duvvuru, D.; Retailleau, P.; Betzer, J.-F.; Marinetti, A. Org. Lett. 2009, 11, 4406.
[51] Shi, Z.; Tong, Q.; Leong, W. W. Y.; Zhong, G. Chem. Eur. J. 2012, 18, 9802.
[52] Shi, Z.; Yu, P.; Loh, T.-P.; Zhong, G. Angew. Chem., Int. Ed. 2012, 51, 7825.
[53] (a) Zhang, X.-N.; Chen, G.-Q.; Dong, X.; Wei, Y.; Shi, M. Adv. Synth. Catal. 2013, 355, 3351. (b) Zhang, X.-N.; Dong, X.; Wei, Y.; Shi, M. Tetrahedron 2014, 70, 2838.
[54] Kao, T.-T.; Syu, S.; Jhang, Y.-W.; Lin, W. Org. Lett. 2010, 12, 3066.
[55] Lee, C.-J.; Jang, Y.-J.; Wu, Z.-Z.; Lin, W. Org. Lett. 2012, 14, 1906.
[56] Chen. K.-W.; Syu, S.; Jang, Y.-J.; Lin, W. Org. Biomol. Chem. 2011, 9, 2098.
[57] Syu. S.; Lee, Y.-T.; Jang, Y.-J.; Lin, W. Org. Lett. 2011, 13, 2970.
[58] Wang, J.; Zhou, R.; He, Z.-R.; He, Z. Eur. J. Org. Chem. 2012, 6033.
/
| 〈 |
|
〉 |