

Chinese Journal of Organic Chemistry >
One-Pot Three-Component Synthesis of New β-Indole Derivatives in Gluconic Acid Aqueous Solution
Received date: 2014-06-18
Revised date: 2014-07-09
Online published: 2014-08-29
Supported by
Project supported by the National Science and Technology Support Program of China (No. 2009BAI75B02), the Innovation Fund Designated for Graduate Students of Jiangxi Province (No. YC2014), and the Outstanding Doctoral Dissertation Cultivation Program of Jiangxi Normal University.
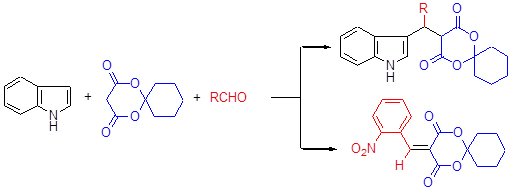
One-pot three-component reactions of aldehydes with indole and 2,2-pentamethylene-1,3-dioxane-4,6-dione afforded thirteen kinds of 5-[(indol-3-yl)-methyl]-2,2-pentamethylene-1,3-dioxane-4,6-dione derivatives (β-indole derivatives) in the presence of gluconic acid aqueous solution in 66.4%~98.5% yields. The advantages of this protocol were mild reaction conditions, wide substrate scope, high yields and benign to environment, which afforded an effective method to synthesize β-indole derivatives.
Yan Nan , Xia Jianhui , Xiong Yunkui , Xiong Bin , Lin Chunhua , Liao Weilin . One-Pot Three-Component Synthesis of New β-Indole Derivatives in Gluconic Acid Aqueous Solution[J]. Chinese Journal of Organic Chemistry, 2014 , 34(12) : 2487 -2492 . DOI: 10.6023/cjoc201406010
[1] Yang, D.-L.; Li, J.-R.; Sun, K.-N.; Lu, H.-Y.; Liu, M.-X.; Shi, D.-X. Chin. J. Org. Chem. 2013, 33, 2341 (in Chinese). (杨德利, 李加荣, 孙克宁, 路红燕, 刘明星, 史大昕, 有机化学, 2013, 33, 2341.)
[2] Tong, G.-J.; Fan, W.; Jiang, B. Chin. J. Org Chem. 2013, 33, 2578 (in Chinese). (佟光进, 范威, 姜波, 有机化学, 2013, 33, 2578.)
[3] Dömling, A. Chem. Rev. 2006, 106, 17.
[4] Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
[5] Francesco, E.; Salvatore, G.; Ornelio, R.; Silvia, T.; Caroline, P.; Massimo, C. Tetrahedron Lett. 2011, 52, 568.
[6] Csaba, N.; Laurent, J.; Janos, S.-T. Tetrahedron Lett. 2000, 56, 5479.
[7] Stéphane, G.; Bérangère, L.; Antonella, F.; Paolode, M.; Janos, S. Tetrahedron 2010, 66, 3065.
[8] Armstrong, E.-L.; Grover, H.-K.; Kerr, M.-A. J. Org. Chem. 2013, 78, 10534.
[9] Oikawa, Y., Hirasawa, H., Yonemitsu, O. Tetrahedron Lett. 1978, 19(20), 1759.
[10] Wang, H.-Y.; Li, L.-L.; Li, W.; Huang, Z.-B.; Shi, D.-Q. Chin. J. Org. Chem. 2013, 33, 1616 (in Chinese). (汪辉员, 李丽丽, 林伟, 黄志斌, 史达清, 有机化学, 2013, 33, 1616.)
[11] Renzetti, A.; Dardennes, E.; Fontana, A.; Maria, P.-D.; Sapi, J.; Gerard, S. J. Org. Chem. 2008, 73, 6824.
[12] Chandrasekhar, S.; Patro, V.; Reddy, G.-P.; Grée, R. Tetrahedron Lett. 2012, 53, 6223.
[13] Qu, Y.-Y.; Ke, F.; Zhou, L.; Li, Z.-K.; Xiang, H.-F.; Wu, D.; Zhou, X.-G. Chem. Commun. 2011, 47, 3912.
[14] Liu, B.-M.; Zhang, D.-L.; Guo, X.-M.; Li, H.-Z. Chin. J. Synth. Chem. 2004, 12, 505 (in Chinese). (刘卉闵, 张冬暖, 果秀敏, 李惠章, 合成化学, 2004, 12, 505.)
[15] Wang, C.; Zhang, Y.-Q.; Li, G.-S.; Li, J.-C.; Li, X.-L. Chin. J. Org. Chem. 2003, 23, 1416 (in Chinese). (王春, 张英群, 李贵深, 李敬慈, 李晓陆, 有机化学, 2003, 23, 1416.)
[16] Sheng, W.-L.; Du, Y.-Y.; Tian, F.-L.; Han, L.-M.; Zhu, N. Chemistry 2012, 75, 1026 (in Chinese). (盛万里, 杜玉英, 田福利, 韩利民, 竺宁, 化学通报, 2012, 75, 1026.)
[17] Zhou, B.-H.; Yang, J.; Li, M.-H.; Gu, Y.-L. Green Chem. 2011, 13, 2204.
[18] Guo, R.-Y.; Wang, P.; Wang, G.-D.; Mo, L.-P.; Zhang, Z.-H. Tetrahedron 2013, 69, 2056.
[19] Yan, N.; Xiong, B.; Liao, W.-L.; Xu, Z.-H. Chin. J. Org. Chem. 2010, 30, 1391 (in Chinese). (严楠, 熊斌, 廖维林, 许招会, 有机化学, 2010, 30, 1391.)
/
| 〈 |
|
〉 |