

Chinese Journal of Organic Chemistry >
Pd-Complex Catalyzed Suzuki Cross-Coupling Reaction of Brominated Salicylaldehyde with Pyridylboronic Acid
Received date: 2014-07-12
Revised date: 2014-08-17
Online published: 2014-09-09
Supported by
Project supported by the National Natural Science Fundation of China (No. J1103303) and the Research Fund of Fuzhou University (No. XRC-1305).
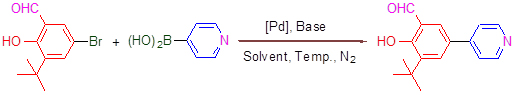
The synthesis of 3-tert-butyl-2-hydroxy-5-(pyridin-4-yl)benzaldehyde by Suzuki cross coupling reaction of 5-bromo-3-tert-butyl-2-hydroxybenzaldehyde with pyridin-4-ylboronic acid was studied by using different catalysts [Pd(dppf)2Cl2, Pd(OAc)2 and Pd(PPh3)4], bases (Na2CO3, NaHCO3, K2CO3, K3PO4, Cs2CO3 and CsF) and solvents (DME/H2O, DMF/H2O and dioxane/H2O) under different temperature of 70, 80 and 100 ℃. A compatible method was developed for the coupling reaction of arylboronic acids (having electron donating or withdrawing group) with aryl bromides (having electron withdrawing group). Under the condition of Pd(PPh3)4, K2CO3, dioxane/H2O (V:V=4:1), 80 ℃, the yield is excellent (97%) and the product separation is easy. The compatibility for aryl bromides with electron donating group is moderate.
Wang Biyu , Ni Peizhong , Fan Jili , Zheng Huidong , Zhao Suying , Bai Zhengshuai . Pd-Complex Catalyzed Suzuki Cross-Coupling Reaction of Brominated Salicylaldehyde with Pyridylboronic Acid[J]. Chinese Journal of Organic Chemistry, 2014 , 34(12) : 2471 -2477 . DOI: 10.6023/cjoc201407024
[1] Baranano, D.; Mann, G.; Hartwig, J. F. Curr. Org. Chem. 1997, 1, 287. (b) Stanforth, S. P. Tetrahedron 1998, 54, 263. (c) Miyaura, N.; Yanagi, T.; Suzuki, A. Synth. Commun. 1981, 11, 513. (d) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. (e) Yan, M.; Feng, X. J. Chin. J. Org. Chem. 2010, 30, 623 (in Chinese). (颜美, 冯秀娟, 有机化学, 2010, 30, 623.)
[2] (a) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. (b) Suzuki, A. Pure Appl. Chem. 1985, 57, 1749. (c) Suzuki, A. Pure Appl. Chem. 1991, 63, 419. (d) Zhang, Y.; Yi, T.; Wang, K.; Fu, J.; Chen, H.; Li, R. X. Chin. J. Org. Chem. 2012, 32, 790 (in Chinese). (张愚, 易韬, 王堃, 付海燕, 陈华, 李瑞祥, 有机化学, 2012, 32, 790.)
[3] Wright, S. W.; Hageman, D. L.; McClure, L. D. J. Org. Chem. 1994, 59, 6095.
[4] Tagata, T.; Nishida, M. J. Org. Chem. 2003, 68, 9412.
[5] Lépine, R.; Zhu, J. Org. Lett. 2005, 7, 2981.
[6] Andereu, I.; Cabedo, N.; Fabis, F.; Cortes, D. Tetrahedron 2005, 61, 8282.
[7] (a) Jesmin, M.; Ali, M. M.; Khanam, J. A. J. Pharm. Sci. 2010, 34(1), 20 (b) Sinha, D.; Tiwari, A. K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A. K. Eur. J. Med. Chem. 2008, 43, 160.
[8] (a) Iolio, A. B.; Gennaro, A. C.; Vianello, E. E. Electrochim. Acta, 1997, 42, 2065. (b) Bastos, R. M. B.; Moreira, J. C.; Farias, P. A. M. Anal. Chim. Acta 2000, 408, 83.
[9] (a) McGarrigle, E. M.; Gilheany, D. G. Chem. Rev. 2005, 105, 1563. (b) Baleizao, C.; Garcia, H. Chem. Rev. 2006, 106, 3987. (c) Decortes, A.; Castilla, A. M.; Kleij, A. W. Angew. Chem., Int. Ed. 2010, 49, 9822. (d) Achard, T. R. J.; Clutterbuck, L. A.; North, M. Synlett 2005, 1828.
[10] Shultz, A. M.; Farha, O. K.; Adhikari, D.; Nguyen, S. T. Inorg. Chem. 2011, 50, 3174.
[11] Huang, Y.; Liu, T.; Cao, R. Inorg. Chem. 2011, 50, 2191.
[12] Zhu, C.; Chen, X.; Yang, Z.; Du, X.; Cui, Y. Chem. Commun. 2013, 49, 7120.
[13] Ryu, E. H.; Lee, J. H.; Lee, Y. S.; Gu, J. M.; Huh, S.; Lee, S. Inorg. Chem. Commun. 2011, 14, 1648.
[14] Morris, G. A.; Nguyen, S. T. Tetrahedron Lett. 2001, 42, 2093.
[15] Kim, W. S.; Lee, K. Y.; Ryu, E. H.; Gu, J. M. Kim, Y.; Lee, S. J.; Huh, S. Eur. J. Inorg. Chem. 2013, 4228.
[16] Lu, G. P.; Voigtritter, K. R.; Cai, C. J. Org. Chem. 2012, 77, 3700.
[17] Wallow, T. I.; Novak. B. M. J. Org. Chem. 1994, 59, 5034.
[18] Xu, C.; Li, Z.; Duan, L. M.; Wang, Z. Q.; Fu, W. J.; Hao, X. Q.; Song, M. P. Transition Met. Chem. 2012, 37, 373.
[19] (a) Kowitz, C.; Wegner, G. Tetrahedron 1997, 53, 15553. (b) Goubet, D.; Meric, P.; Dormoy, J. R.; Moreau, P. J. Org. Chem. 1999, 64, 4516.
[20] (a) Aliprantis, A. O.; Canary, J. W. J. Am. Chem. Soc. 1994, 116, 6985. (b) Martin, A. R.; Yang, Y. Acta Chem. Stand. 1993, 47, 221.
[21] Tan, R.; Yin, D.; Yu, N.; Tao, L.; Fu, Z.; Yin, D. J. Mol. Catal. A: Chem. 2006, 259, 125.
[22] Cavazzini, M.; Manfredi, A.; Montanari, F.; Quici, S.; Pozzi, G.; Eur. J. Org. Chem. 2001, 24, 4639.
/
| 〈 |
|
〉 |