

Chinese Journal of Organic Chemistry >
Iron-Catalyzed Oxidative Phosphonation of α-sp3-C—H Bonds of N-Aryl Tetrahydroisoquinolines with Air as Oxidant
Received date: 2014-08-21
Revised date: 2014-09-28
Online published: 2014-10-09
Supported by
Project supported by the National Natural Science Foundation of China (No.21302067) and the National Natural Science Foundation of Jiangsu Province (No.BK20130120).
An efficient cross-dehydrogenative-coupling (CDC) between sp3-C—H bond adjacent to a nitrogen atom of tertiary amine and H—P bonds of dialkyl phosphites and diaryl phosphate was developed using FeCl3 as catalyst and air as oxidant under mild reaction conditions. The safe, convenient, environmental and efficiently benign to synthesize a series of biologically important α-aminophosphonates.
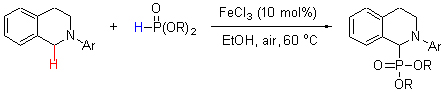
Zhang Yan , Luo Sha , Feng Bainian . Iron-Catalyzed Oxidative Phosphonation of α-sp3-C—H Bonds of N-Aryl Tetrahydroisoquinolines with Air as Oxidant[J]. Chinese Journal of Organic Chemistry, 2014 , 34(11) : 2249 -2254 . DOI: 10.6023/cjoc201408025
[1] (a) Li, Z.; Li, C. J. J. Am. Chem. Soc. 2005, 127, 3672.(b) Basle, C.; Li, C. J. Green Chem. 2007, 9, 1047. (c) Boess, E.; Sureshkumar, D.; Sud, A.; Wirtz, C.; Fares, C.; Klussmann, M. J. Am. Chem. Soc. 2011, 133, 8106.(d) Xiao, T. B.; Li, L. Y.; Lin, G. L.; Mao, Z. W.; Zhou, L. Org. Chem. 2014, 16, 4232.(e) Zheng, Z. S.; Dian, L. Y.; Yuan, Y. C.; Zhang, N. D.; Du, Y. F.; Zhao, K. J. Org. Chem. 2014, 79, 7451.
[2] (a) Han, W.; Ofial, A. R. Chem. Commun. 2009, 5024.(b) Liu, P.; Liu, Y.; Wong, E. L. M.; Xiang, S.; Che, C. M. Chem. Sci. 2011, 2, 2187.(c) Yu, L.; Wang, M.; Wang, L. Tetrahedron 2014, 70, 5391.
[3] (a) Yoo, W. J.; Li, C. J. Top. Curr. Chem. 2009, 292, 281. (b) Rout, S. K.; Guin, S.; Ali, W.; Gogoi, A.; Patel, B. K. Org. Lett. 2014, 16, 3086. (c) Yoo, W. J.; Kobayashi, S. Green Chem. 2014, 16, 2438.(d) Yan, G. B.; Yu, J.; Zhang, L. Chin. J. Org. Chem. 2012, 32, 294 (in Chinese).(严国兵, 于健, 张玲, 有机化学, 2012, 32, 294.)(e) Liu, W.; Bi, Y. L. Chin. J. Org. Chem. 2012, 32, 1041 (in Chinese). (刘伟, 毕艳兰, 有机化学, 2012, 32, 1041.)(f) Pan, F.; Shi, Z. J. Acta Chim. Sinica 2012, 71, 1679 (in Chinese).(潘菲, 施章杰, 化学学报, 2012, 71, 1679.) (g) Xu, Q.; Zhao, C. Q.; Zhou, Y. B.; Yi, S. F.; Han, L. B. Chin. J. Org. Chem. 2012, 32, 1761 (in Chinese).(徐清, 赵长秋, 周永波, 尹双凤, 韩立彪, 有机化学, 2012, 32, 1761.)
[4] (a) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.(b) Sun, C. L.; Li, B, J,; Shi, Z. J. Chem. Rev. 2011, 111, 1293.(c) Zhang, Y.; Luo, S.; Zhu, C. J. Chin. J. Org. Chem. 2012, 32, 2073 (in Chinese).(张艳, 罗莎, 朱成建, 有机化学, 2012, 32, 2073.)(d) Zhang, Y.; Zhu, C. J. Chin. J. Org. Chem. 2012, 32, 2090 (in Chinese).(张艳, 朱成建, 有机化学, 2012, 32, 2090.)
[5] (a) Liu, C.; Zhang, H.; Shi, W.; Li, A. Chem. Rev. 2011, 111, 1780. (b) Li, B. J.; Shi, Z. J. Chem. Soc. Rev. 2012, 41, 5588. (c) Zhang, B.; Guan, H. X.; Liu, B.; Shi, B. F. Chin. J. Org. Chem. 2014, 34, 1487 (in Chinese). (张博, 管晗曦, 刘斌, 史炳锋, 有机化学, 2014, 34, 1487.) (d) Li, H.; Ding, C. H.; Xu, B.; Hou, X. L. Acta Chim. Sinica 2014, 72, 765 (in Chinese).(李浩, 丁昌华, 许斌, 侯雪龙, 化学学报, 2014, 72, 765.)
[6] (a) Mihovilovic, M. D.; Schnuerch, M. ChemCatChem 2014, 6, 2194. (b) Labre, F.; Gimbert, Y.; Bannwarth, P.; Olivero, S.; Dunach, E.; Chavant, P. Y. Org. Lett. 2014, 16, 2366.(c) Xu, T.; Cheung, C. W.; Hu, X. Angew. Chem., Int. Ed. 2014, 53, 4910. (d) Li, Z. P.; Cao, L.; Li. C. J. Angew. Chem., Int. Ed. 2007, 46, 6505. (e) He, R. Y.; Huang, Z. T.; Zheng, Q. Y.; Wang, C. Y. Angew. Chem., Int. Ed. 2014, 53, 4950.
[7] (a) Jia, F.; Li, Z. P. Org. Chem. Front. 2014, 1, 194. (b) Asako, S.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2013, 135, 17755. (c) Chiranjeevi, B.; Koyyada, G.; Prabusreenivasan, S.; Kumar, V.; Sujitha, P.; Kumar, C. G.; Sridhar, B.; Shaik, S.; Chandrasekharam, M. RSC Adv. 2013, 3, 16475. (d) Guo, X. W.; Yu, R.; Li, H. J.; Li, Z. P. J. Am. Chem. Soc. 2009, 131, 17387. (e) Zhou, B. W.; Chen, H.; Wang, C. Y. J. Am. Chem. Soc. 2013, 135, 1264.
[8] (a) Malhotra, S.; Seng, P. S.; Koenig, S. G.; Deese, A. J.; Ford, K. A. Org. Lett. 2013, 15, 3698. (b) Lu, L. Q.; Li, Y. H.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 8382. (c) Li, Y. M.; Ma, L. N.; Jia, F.; Li, Z. P. J. Org. Chem. 2013, 78, 5638. (d) Wang, C. Y. Synlett 2013, 24, 1606.
[9] (a) Manabe, K.; Kobayashi, S. Chem. Commun. 2000, 669.(b) Yadav, J. S.; Reddy, B. V. S.; Raj, K. S.; Reddy, K. B.; Prasad, A. R. Synthesis 2001, 2277.
[10] (a) Saidi, M. R.; Azizi, N. Synlett 2002, 1347. (b) Akiyama, T.; Sanada, M.; Fuchibe, K. Synlett 2003, 1463.
[11] (a) Han, W.; Ofial, R. Chem. Commun. 2009, 6023. (b) Hari, D. P.; Konig, B. Org. Lett. 2011, 13, 3852.
[12] (a) Xue, Q. C.; Xie, J.; Jin, H. M.; Cheng, Y. X.; Zhu, C. J. Org. Biomol. Chem. 2013, 11, 1606. (b) Rueping, M.; Zhu, S. Q.; Koenigs, R. M. Chem. Commun. 2011, 47, 8679. (c) Dhineshkumar, J.; Lamani, M.; Alagiri, K.; Prabhu, K. R. Org. Lett. 2013, 15, 1092.
[13] (a) Xie, J.; Li, H. M.; Xue, Q. C.; Cheng, Y. X.; Zhu, C. J. Adv. Synth. Catal. 2012, 354, 1646. (b) Alagiri, K.; Devadig, P.; Prabhu, K. R. Chem. Eur. J. 2012, 18, 5160. (c) Alagiri, K.; Devadig, P.; Prabhu, K. R. Tetrahedron Lett. 2012, 53, 1456. (d) Wang, H. L.; Li, X. C.; Wu, F.; Wan, B. S. Tetrahedron Lett. 2012, 53, 681.
[14] (a) Zhang, Y.; Peng, H.; Zhang, M.; Cheng, Y. X.; Zhu, C. J. Chem. Commun. 2011, 47, 2354. (b) Zhang, Y.; Zhu, C. J. Catal. Commun. 2012, 28, 134.(c) Zhang, Y.; Feng, B. N.; Zhu, C. J. Org. Biomol. Chem. 2012, 10, 9137.
[15] (a) Huo, C. D.; Wang, C.; Wu, M. X.; Jia, X. D.; Yuan, Y.; Xie, H. S. Org. Biomol. Chem. 2014, 12, 3123.(b) Basle O.; Li, C. J. Chem. Commun. 2009, 4124.
[16] Li, Z. P.; Li, C. J. J. Am. Chem. Soc. 2005, 127, 3672.
/
| 〈 |
|
〉 |