

Chinese Journal of Organic Chemistry >
Solvent-Free One-Pot Three-Component Synthesis of 5-[(Indol-3-yl)-methyl]-2,2-butylidene-1,3-dioxane-4,6-dione Derivatives with B(OH)3 as Catalyst
Received date: 2014-07-09
Revised date: 2014-10-10
Online published: 2014-10-13
Supported by
Project supported by the National Science and Technology Project (No. 2001BA323C) and the Graduate Innovation Foundation of Jiangxi Province (No. YC10A51).
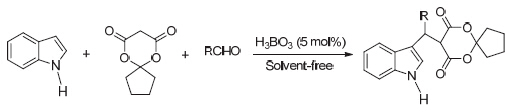
Nine kinds of 5-[(indol-3-yl)-methyl]-2,2-butylidene-1,3-dioxane-4,6-dione derivatives were synthesized by the three-component one-pot reaction of indole with aldehydes and 2,2-butylidene-1,3-dioxane-4,6-dione in the presence of boric acid under solvent-free. The results indicated that the yields ranged from 68.6% to 91.3%, when using 5 mol% boric acid and reacting at 60 ℃ for 30~90 min. Furthermore, a proposed reaction mechanism for the reaction catalyzed by boric acid was speculated. The main advantages of the present procedure were milder conditions, shorter reaction time and higher yields. Further study showed that boric acid was environmentally friendly and reused for six times without any noticeable decrease in the catalytic activity.
Lin Chunhua , Xu Zhaohui , Liao Weilin , Qiu Zengye , Xia Jianhui . Solvent-Free One-Pot Three-Component Synthesis of 5-[(Indol-3-yl)-methyl]-2,2-butylidene-1,3-dioxane-4,6-dione Derivatives with B(OH)3 as Catalyst[J]. Chinese Journal of Organic Chemistry, 2015 , 35(1) : 212 -216 . DOI: 10.6023/cjoc201407013
[1] Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796.
[2] Yang, D. L.; Li, J. R.; Sun, K. N. Chin. J. Org. Chem. 2013, 33, 2341 (in Chinese). (杨得利, 李家荣, 孙克宁, 有机化学, 2013, 33, 2341.)
[3] Yi, Z. K.; Tian, S. B. Chin. J. Org. Chem. 2014, 34, 387 (in Chinese). (伊志奎, 田拴宝, 有机化学, 2014, 34, 387.)
[4] Chen, Z. W.; Bi, J. H.; Su, W. K. Chin. J. Chem. 2013, 31, 507.
[5] Tong, G. J.; Xu, H. W.; Fan, W.; Jiang, B.; Wang, S. L.; Tu, S. J. Chin. J. Chem. 2013, 31, 1034.
[6] Francesco, E.; Salvatore, G.; Ornelio, R. Tetrahedron Lett. 2011, 52, 568.
[7] Janos, G.; Gabor, P.; Tamas, N. J. Comb. Chem. 2005, 7, 530.
[8] Marcelo, V. H.; Ingo, K.; Nayanede, S. ACS Comb. Sci. 2012, 14, 434.
[9] Janos, G.; Gyorgy, D.; Ferenc, D. QSAR Comb. Sci. 2006, 25, 439.
[10] Yuji, O.; Hitoshi, H.; Osamu, Y. Tetrahedron Lett. 1978, 20, 1759.
[11] Csaba, N.; Laurent, J.; Janos, S. Tetrahedron 2000, 56, 5479.
[12] Andrea, R.; Emmanuel, D.; Antonella, F. J. Org. Chem. 2008, 73, 6824.
[13] Srivari, C.; Vidyavathi, P.; Gangireddy, P. Tetrahedron Lett. 2012, 53, 6223.
[14] Eric, F.; Aaron, M. D.; Bryan, A. K. J. Org. Chem. 2006, 71, 409.
[15] Dumas, A. M.; Seed, A.; Zorzitto, A. K.; Fillion, E. Tetrahedron Lett. 2007, 48, 7072.
[16] Goutam, B.; Suvankar, C. RSC Adv. 2014, 4, 7380.
[17] (a) Ganguly, N. C.; Roy, S.; Mondal, P. Synth. Commun. 2014, 44, 433. (b) Halimehjnai, A. Z.; Hosseyni, S.; Gholami, H.; Hashemi, M. M. Synth. Commun. 2013, 43, 191. (c) Meshram, H. M.; Rao, N. N.; Thakur, P. B.; Reddy, B. C.; Ramesh, P. Indian J. Chem. 2013, 52, 814. (d) Nguyen, T. B.; Sorres, J.; Tran, M. Q.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2012, 14, 3202. (e) Singh, M.; Fatma, S.; Ankit, P.; Singh, S. B.; Singh, J. Tetrahedron Lett. 2014, 55, 525.
[18] Kondaiah, G. C.; Reddy, L. A.; Babu, K. S.; Gurav, V. M.; Huge, K. G.; Bandichhor, R.; Reddy, P. P.; Bhattachhrya, A.; Anand, V. R. Tetrahedron Lett. 2008, 49, 106.
[19] Liu, L. T.; Zhang, Y.; Jiao, J.; Yang, F. Y.; Lu, H. J. Acta Chim. Sinica 2013, 71, 535 (in Chinese). (刘丽婷, 张莹, 焦竟, 杨芃原, 陆豪杰, 化学学报, 2013, 71, 535.)
[20] Zorkun, I. S.; Sarae, S.; Celebi, S. Bioorg. Med. Chem. 2006, 14, 852.
[21] Chaudhuri, M. K.; Hussain, S. J. Mol. Catal. A: Chem. 2007, 269, 214.
[22] Xu, Z. H.; Lin, C. H.; Xia, J. H. Heterocycl. Lett. 2013, 3, 319.
[23] Wang, C.; Zhang, Y. Q.; Li, G. S.; Li, J. C.; Li, X. L. Chin. J. Org. Chem. 2003, 23, 1416 (in Chinese). (王春, 张英群, 李贵深, 李敬慈, 李晓陆, 有机化学, 2003, 23, 1416.)
[24] Xu, Z. H. Chin. J. Org. Chem. 2014, 34, 1687 (in Chinese). (许招会, 有机化学, 2014, 34, 1687.)
[25] Xu, Z. H.; Li, C. H. Chin. J. Org. Chem. 2013, 33, 1540 (in Chinese). (许招会, 林春花, 有机化学, 2013, 33, 1540.)
[26] Yan. N.; Xiong, B.; Liao, W. L.; Xu, Z. H. Chin. J. Org. Chem. 2010, 30, 1391 (in Chinese). (严楠, 熊斌, 廖维林, 许招会, 有机化学, 2010, 30, 1391.)
/
| 〈 |
|
〉 |