

Chinese Journal of Organic Chemistry >
Theoretical Investigation on Firefly Oxyluciferin Analogs Bearing an Amino Group Used as Organic Light-Emitting Diodes Materials
Received date: 2014-09-03
Revised date: 2014-10-13
Online published: 2014-10-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21473071, 21173099, 21363012, 51363012, 51374117), the National Key Basic Research Program (973 Program, No. 2013CB834801), the Special Funding to Basic Scientific Research Projects for Central Colleges, the Applied Basic Research Plans Program of Yunnan Province (No. 2011FZ040), the Scientific Research Fund of Yunnan Provincial Education Department (No. 2012Y545), the Training Foundation for Talents of Kunming University of Science and Technology (No. KKSY201232040) and the Foundation of State Key Laboratory of Theoretical and Computational Chemistry.
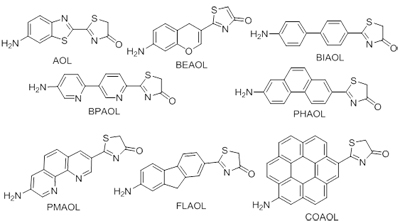
Firefly oxyluciferin has great potential application in organic light-emitting devices, because of the high efficiency and wide range of multicolor light from green to red. To gain an insight into the structure-property relationships, a set of firefly oxyluciferin analogs bearing an amino group with benzopyran, biphenyl, bipyridine, phenanthrene, phenanthroline, fluorine and coronene instead of benzothiazole ring were designed. In this study, a systematic investigation into them was carried out using the density functional theory and time-dependent density functional theory methods. The calculated values show that bipyridylaminooxyluciferin (BPAOL) and orthophenanthrolylaminooxyluciferin (PMAOL) with nitrogen atom have smaller the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energies, larger ionization potential (IP) and electron affinity (EA) values than biphenylaminooxyluciferin (BIAOL) and phenanthrylaminooxyluciferin (PHAOL) with carbon atom. Compound aminooxyluciferin (AOL), BIAOL, BPAOL, PHAOL, PMAOL, fluorenylaminooxyluciferin (FLAOL) and coronenylaminooxyluciferin (COAOL) can be used as electron-injection/transporting materials. AOL, PHAOL, FLAOL and COAOL can be used as blue light-emitting materials. These results indicate that the firefly oxyluciferin analogs bearing an amino group have many interesting properties and are good candidates for optoelectronic ap-plication.
Min Chungang , Li Zuosheng , Cui Xiaoying , Yang Xikun , Huang Shaojun , Wang Shaohua , Ren Aimin . Theoretical Investigation on Firefly Oxyluciferin Analogs Bearing an Amino Group Used as Organic Light-Emitting Diodes Materials[J]. Chinese Journal of Organic Chemistry, 2015 , 35(2) : 432 -438 . DOI: 10.6023/cjoc201409003
[1] Shen, P. Y.; Wu, S. H.; Huang, Y. T.; Wei, Y. Res. Chem. Intermed. 2014, 40, 2199.
[2] Choi, A. Y.; Yamaguchi, T.; Han, C.-H. Res. Chem. Intermed. 2013, 39, 1571.
[3] Han, L. Z.; Wang, Z.; Hua, Y. J.; Ren, A. M.; Liu, Y. L.; Liu, P. J. Acta Chim. Sinica 2012, 70, 579 (in Chinese). (韩立志, 王卓, 华英杰, 任爱民, 刘艳玲, 刘朋军, 化学学报, 2012, 70, 579.)
[4] Zhao, L. L.; Jiu, Y. D.; Wang, J. Y.; Zhang, X. W.; Lai, W. Y.; Huang, W. Acta Chim. Sinica 2013, 71, 1248 (in Chinese). (赵玲玲, 酒元达, 王建云, 张新稳, 赖文勇, 黄维, 化学学报, 2013, 71, 1248.)
[5] Huang, B.; Tang, J. N.; Jiang, W.; Yang, W.; Ban, X. X.; Sun, Y. M. Chin. J. Org. Chem. 2013, 33, 1395 (in Chinese). (黄斌, 唐霁楠, 蒋伟, 杨文, 班鑫鑫, 孙岳明, 有机化学, 2013, 33, 1395.)
[6] Wang, W. Y.; Ma, N. N.; Sun, S. L.; Qiu, Y. Q. Organometallics 2014, 33, 3341
[7] Song, H. J.; Zhang, M. Y.; Yu, H. L.; Wang, C. H.; Zou, H. Y.; Ma, N. N.; Qiu, Y. Q. Comput. Theor. Chem. 2014, 1031, 7.
[8] Gong, Y.; Zhang, Y.; Yuan, W. Z.; Sun, J. Z.; Zhang, Y. J. Phys. Chem. C 2014, 118, 10998.
[9] Shirota, Y. J. Mater. Chem. 2005, 15, 75.
[10] Shen, X. Y.; Yuan, W. Z.; Liu, Y.; Zhao, Q.; Lu, P.; Ma, Y.; Williams, I. D.; Qin, A.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2012, 116, 10541.
[11] Shen, X. Y.; Wang, Y. J.; Zhao, E.; Yuan, W. Z.; Liu, Y.; Lu, P.; Qin, A.; Ma, Y.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2013, 117, 7334.
[12] Kim, F. S.; Guo, X.; Watson, M. D.; Jenekhe, S. Adv. Mater. 2010, 22, 478.
[13] Lin, T.-C.; He, G. S.; Zheng, Q.; Prasad, P. N. J. Mater. Chem. 2006, 16, 2490.
[14] Grabowski, Z. R.; Rotkiewicz, K.; Rettig, W. Chem. Rev. 2003, 103, 3899.
[15] Liu, Y. J.; Vico, L. D.; Lindh, R. J. Photochem. Photobiol. A: Chem. 2008, 194, 261.
[16] Cai, D.; Marques, M. A. L.; Milne, B. F.; Nogueira, F. J. Phys. Chem. Lett. 2010, 1, 2781.
[17] Ando, Y.; Niwa, K.; Yamada, N.; Enomoto, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Nat. Photonics 2008, 2, 44.
[18] Min, C. G.; Leng, Y.; Yang, X. K.; Huang, S. J.; Ren, A. M. Chem. J. Chin. Univ. 2014, 35, 564 (in Chinese). (闵春刚, 冷艳, 杨喜昆, 黄绍军, 任爱民, 高等学校化学学报, 2014, 35, 564.)
[19] Ishida, A.; Yoshikawa, T.; Kamidate, T. Anal. Biochem. 2003, 316, 127.
[20] Branchini, B. R.; Ablamsky, D. M.; Rosenman, J. M.; Uzasci, L.; Southworth, T. L.; Zimmer, M. Biochemistry 2007, 46, 13847.
[21] Viviani, V. R.; Oehlmeyer, T. L.; Arnoldi, F. G. C.; Brochetto-Braga, M. R. Photochem. Photobiol. 2005, 81, 843.
[22] Takakura, H.; Sasakura, K.; Ueno, T.; Urano, Y.; Terai, T.; Hanaoka, K.; Tsuboi, T.; Nagano, T. Chem. Asian J. 2010, 5, 2053.
[23] Li, Z. S.; Min, C. G.; Ren, A. M.; Zou, L. Y.; Xu, Z. J. Photochem. Photobio. A 2012, 243, 7-16
[24] Chu, T. Y.; Ho, M. H.; Chen, J. F.; Chen, C. H. Chem. Phys. Lett. 2005, 415, 137.
[25] Li, X.; Wang, X.; Gao, J.; Yu, X.; Wang H. Chem. Phys., 2006, 326, 390.
[26] The electron and hole transporting properties are related to the energies of HOMO and LUMO, IP(a) and EA(a) values as organic light-emitting diodes materials. In this paper, the HOMO and LUMO energies, IP(a) and EA(a) values of the studied molecules are compared with the vales of BNPB molecule in reference 25. B3LYP/6-31G(d) method was used in reference 25, and MPW3PBE/6-31+G(d) method is use in this paper. In fact, the difference in functional and basis sets may lead to different computational results. Therefore, the HOMO and LUMO energies, IP(a) and EA(a) values for BNPB were recalculation by MPW3PBE/ 6-31+G(d) method.
[27] Nelsen, S. F.; Blomgren. F. J. J. Org. Chem., 2001, 66, 6551.
[28] Hutchison, G. R.; Ratner, M. A.; Marks, T. J. J. Am. Chem. Soc. 2005, 127, 2339.
[29] Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
[30] Adamo, C.; Barone, V. J. Chem. Phys. 1998, 108, 664
[31] Min, C. G.; Leng, Y.; Yang, X. K.; Ren, A. M.; Cui, X. Y.; Xu, M. L.; Wang, S. H. Chem. Res. Chin. Univ. 2013, 29, 982.
[32] Ren, X. F.; Ren, A. M.; Feng, J. K.; Sun, C. C. J. Photochem. Photobiol. A: Chem. 2009, 203, 92.
[33] Liu, Y. L.; Feng, J. K.; Ren, A. M. J. Comput. Chem. 2007, 28, 2500.
[34] Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999.
[35] Cramer, C. J.; Truhlar, D. G. Chem. Rev. 1999, 99, 2161.
[36] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009.
/
| 〈 |
|
〉 |