

Chinese Journal of Organic Chemistry >
Synthesis of a Tubulin Assembly Inhibitors OXi8006
Received date: 2014-08-29
Revised date: 2014-10-21
Online published: 2014-10-23
Supported by
Project supported by the National Natural Science Foundation of China (No. 81230090), the Shanghai Leading Academic Discipline Project (No. B906).
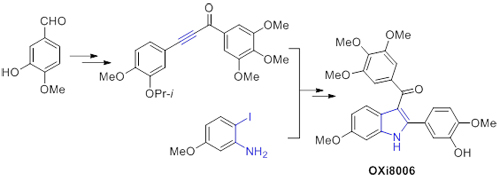
2-(3'-Hydroxy-4'-methoxyphenyl)-3-(3",4",5"-trimethoxybenzoyl)-6-methoxyindole (OXi8006) was found to be a strong inhibitors of tubulin assembly and showed excellent anticancer activity (IC50=1.1 μmol/L). Reported synthetic method of OXi8006 suffered from some drawbacks, such as long steps and low overall yields. Therefore, developing more efficient and practical protocols to synthesize OXi8006 is highly desirable. Firstly, we prepared aryl acetylene from commercially available isovanillin in three steps. Then the aryl acetylene was converted to diaryl acetylene ketone by reacting with 3,4,5-trimethoxybenzaldehyde via nucleophilic addition and oxidation reaction. The key step of 2-aryl-3-aroyl-indole construction was carried out between diaryl acetylenic ketone and o-iodo aniline by performing aza-Michael addition and subsequent intramolecular Heck reaction. The yield of developed synthesis over six steps is 20%.
Key words: tubulin assembly inhibitors; OXi8006; aza-Michael addition; Heck reaction
Liu Chunting , Bi Kaijian , Chang Wanlin , Ye Ji , Zhang Weidong , Sun Qingyan . Synthesis of a Tubulin Assembly Inhibitors OXi8006[J]. Chinese Journal of Organic Chemistry, 2015 , 35(2) : 484 -489 . DOI: 10.6023/cjoc201408037
[1] (a) Lin, C. M.; Singh, S. B.; Chu, P. S.; Dempcy, R. O.; Schmidt, J. M.; Pettit, G. R.; Hamel, E. Mol. Pharmacol. 1988, 34, 200.
(b) Liou, J.-P.; Chang, Y.-L.; Kuo, F. M.; Chang, C. W.; Tseng, H. Y.; Wang, C. C.; Yang, Y. N.; Chang, J. Y.; Lee, S. S.; Hsieh, H. P. J. Med. Chem. 2004, 47, 4247.
(c) Li, H.-X.; Liu, X.-R.; Zhang, W.-P.; Zhang, C.; Sun, H.-B. Chin. J. New. Drugs Clin. Rem. 2010, 29(11), 816 (in Chinese). (李洪雪, 刘晓蓉, 张文萍, 张仓, 孙宏斌, 中国新药与临床杂志, 2010, 29(11), 816.)
[2] (a) MacDonough, M. T.; Strecker, T. E.; Hamel, E.; Hall, J. J.; Chaplin, D. J.; Trawick, M. L.; Pinney, K. G. Bioorg. Med. Chem. 2013, 21, 6831.
(b) Hadimani, M. B.; MacDonough, M. T.; Ghatak, A.; Strecker, T. E.; Lopez, R.; Sriram, M.; Nguyen, B. L.; Hall, J. J.; Kessler, R. J.; Shirali, A. R.; Liu, L.; Garner, C. M.; Pettit, G. R.; Hamel, E.; Chaplin, D. J.; Mason, R. P.; Trawick, M. L.; Pinney, K. G J. Nat. Prod. 2013, 76, 1668.
(c) Flynn, B. L.; Hamel, E. WO 2002060872, 2002 [Chem. Abstr. 2002, 137, 154849].
[3] Hadimani, M.; Mejia, M.; Pinney, K.; Wang, F. US 20070082872, 2007 [Chem. Abstr. 2007, 146, 395269].
[4] Flynn, B. L.; Hamel, E.; Jung, M. K. J. Med. Chem. 2002, 45, 2670.
[5] Yuan, H.; Bi, K.-J.; Li, B.; Yue, R. C.; Ye, J.; Shen, Y. H.; Shan, L.; Jin, H. Z.; Sun, Q. Y.; Zhang, W. D. Org. Lett. 2013, 15, 4742.
[6] Beugelmans, R.; Roussi, G.; Zamora, E. G.; Carbonelle, A. C. Tetrahedron 1999, 55, 5089.
[7] Banwell, M. G.; Flynn, B. L. WO 9850365, 1998 [Chem. Abstr. 1998, 130, 3974]
[8] (a) Ridley, C. P.; Reddy, M.; Rocha, G.; Bushman, F. D.; Faulkner, D. J. Bioorg. Med. Chem. 2002, 10, 3285.
(b) Rosiak, A.; Frey, W.; Christoffers, J. Eur. J. Org. Chem. 2006, 17, 4044.
[9] (a) Kerr, D. J.; Hamel, E.; Jung, M. K.; Flynn, B. L. Bioorg. Med. Chem. 2007, 15, 3290.
(b) Yang, X.-M.; Zhang, Y.-S.; Yao, Y.; Tao, Y.-H. Chin. J. Org. Chem. 2014, 34, 1458 (in Chinese). (杨晓梅, 张玉顺, 姚赟, 陶云海, 有机化学, 2014, 34, 1458.)
[10] (a) Sakamoto, T.; Nagano, T.; Kondo, Y.; Yamanaka, H. Synthesis 1990, 215.
(b) Cai, S.; Yang, K.; Wang, D. Z. Org. Lett. 2014, 16, 2606.
(c) Würtz, S.; Rakshit, S.; Neumann, J. J.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2008, 47, 7230.
(d) Batail, N.; Dufaud, V.; Djakovitch, L. Tetrahedron 2011, 52, 1916.
[11] Flynn, B. L.; Verdier-Pinard, P.; Hamel, E. Org. Lett. 2001, 3, 651.
/
| 〈 |
|
〉 |