

Chinese Journal of Organic Chemistry >
Domino Synthesis of Novel Chromene-Indanedione Derivatives
Received date: 2014-08-27
Revised date: 2014-10-24
Online published: 2014-11-07
Supported by
Project supported by the Educational Commission of Hubei Province (No. D20142501) and the National Natural Science Foundation of China (No. 21102042).
A simple method for the synthesis of novel chromene-indanedione derivatives (3) has been developed by the reaction of 2-hydroxychalcones (1) with 1,3-indanedione (2) in reflux toluene under catalyst-free conditions. All the compounds were characterized by means of 1H NMR, 13C NMR, IR and HRMS. This reaction probably underwent a domino Michael addition/cyclization/oxidation process. The UV-Vis spectroscopy indicated that the maximum absorption wavelengths of the products 3a~3h were at the range of 464~482 nm.
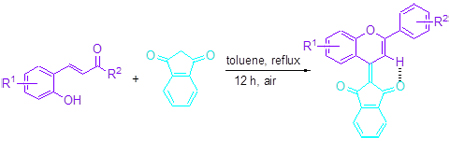
Key words: chromene; 1,3-indanedione; 2-hydroxychalcones; domino reaction
Rao Yin , Liu Meilin , Yin Guodong . Domino Synthesis of Novel Chromene-Indanedione Derivatives[J]. Chinese Journal of Organic Chemistry, 2015 , 35(3) : 719 -723 . DOI: 10.6023/cjoc201408032
null
/
| 〈 |
|
〉 |