

Chinese Journal of Organic Chemistry >
Synthesis of a Carbene Complex and Its Application in Suzuki Reaction
Received date: 2014-08-25
Revised date: 2014-11-04
Online published: 2014-11-13
Supported by
Project supported by the National Natual Science Foundation of China (No. 21402067).
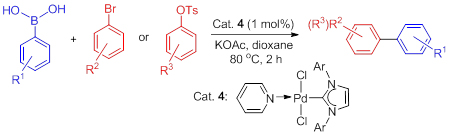
A palladium N-heterocyclic (NHC) complex has been synthesized and successfully developed as an efficient catalyst for the Suzuki coupling reaction of aryl bromides and 4-toluene sulfonic acid aromatic esters with aryl boronic acids. The catalytic reaction proceeded smoothly in dioxane with potassium acetate as the additive at 80 ℃ in an air atmosphere, while the yield of the target product is excellent and the catalyst can be synthesized easily.
Key words: Suzuki coupling reaction; carbene; palladium complex; synthesis
Tang Yan, Yang Feifei, Nie Shipeng, Wang Lin Luo Zhibin, Lu Hongfei . Synthesis of a Carbene Complex and Its Application in Suzuki Reaction[J]. Chinese Journal of Organic Chemistry, 2015 , 35(3) : 705 -711 . DOI: 10.6023/cjoc201408028
[1] (a) Wei, S.-H.; Wang, C.; Wang, J.-W.; Cheng, K. Chin. J. Org. Chem. 2013, 33, 2402 (in Chinese). (韦珊红, 王晨, 王建伟, 程凯, 有机化学, 2013, 33, 2402.) (b) Chu, W.-Y.; Wang, M.; Li, X.-M.; Hou, Y.-J.; Sun, Z.-Z. Chin. J. Org. Chem. 2012, 32, 1666 (in Chinese). (初文毅, 王熳, 李新民, 侯艳君, 孙志忠, 有机化学, 2012, 32, 1666.) (c) Zhang, Y.; Yi, T.; Wang, K.; Fu, H.-Y.; Chen, H.; Li, R.-X. Chin. J. Org. Chem. 2012, 32, 790 (in Chinese). (张愚, 易韬, 王堃, 付海燕, 陈华, 李瑞祥, 有机化学, 2012, 32, 790.) (d) Liu, N.; Liu, C.; Jin, Z.-L. Chin. J. Org. Chem. 2012, 32, 860 (in Chinese). (刘宁, 刘春, 金子林, 有机化学, 2012, 32, 860.)
[2] Corbet, J. P.; Mignani, G. Chem. Rev. 2006, 106, 2651.
[3] Wu, X. F.; Anbarasan, Dr. P.; Neumann, Dr. H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9047.
[4] (a) Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 2002, 41, 4176. (b) Chung, K. H.; So, C. M.; Wong, S. M.; Luk, C. H.; Zhou, Z. Y.; Lau, C. P.; Kwong, F. Y. Chem. Commun. 2012, 48, 1967. (c) Tang, W. J.; Keshipeddy, S.; Zhang, Y. D.; Wei, X. D.; Savoie, J.; Patel, N. D.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2011, 13, 1366. (d) Leowanawat, P.; Zhang, N.; Safi, M.; Hoffman, D. J.; Fryberger, M. C.; George, A.; Percec, V. J. Org. Chem. 2012, 77, 2885. (e) Wang, Z. Y.; Chen, G. Q.; Shao, L. X. J. Org. Chem. 2012, 77, 6608. (f) Dubbaka, S. R.; Vogel, P. Org. Lett. 2004, 6, 95.
[5] Zim, D.; Lando, V. R.; Duponl, J.; Monteiro, A. L. Org. Lett. 2001, 3, 3049.
[6] Roy, A. H.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 8704.
[7] Zim, D.; Lando, V. R.; Dupont, J.; Monteiro, A. L. Org. Lett. 2001, 3, 3049.
[8] Herrmann, W. A.; Elison, M.; Fischer, J.; Kocher. C.; Artus, G. R. J. Angew. Chem., Int. Ed. 1995, 34, 2371.
[9] Skell, P. S.; Sandier, S. R. J. Am. Chem. Soc. 1958, 80, 2024.
[10] Wanzlick, H. W.; Schnherr, H. J. Angew. Chem., Int. Ed. Engl. 1968, 7, 141.
[11] (a) Ackermann, L.; Althammer, A. Org. Lett. 2006, 8, 3457. (b) Tang, Z. Y.; Spinella, S.; Hu, Q. S. Tetrahedron Lett. 2006, 47, 2427. (c) Gao, H.; Li, Y.; Zhou, Y. G.; Han, F. S.; Lin, Y. J. Adv. Synth. Catal. 2011, 353, 309. (d) Tang, Z. H.; Hu, Q. S. Adv. Synth. Catal. 2004, 346, 1635. (e) Xing, C. H.; Lee, J. R.; Tang, Z. Y.; Zheng, J. R.; Hu, Q. S. Adv. Synth. Catal. 2011, 353, 2051. (f) Tang, Z. Y.; Hu, Q. S. J. Am. Chem. Soc. 2004, 126, 3058.
[12] (a) Brenner, E.; Matt, D.; Henrion, M.; Teci, M.; Toupet, L. Dalton Trans. 2011, 40, 9889. (b) Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A. M.; Garg, N. K.; Percec, V. Chem. Rev. 2011, 111, 1346. (c) Chen, M. T.; Vicic, D. A.; Turner, M. T.; Navarro, O. Organometallics 2011, 30, 5052.
[13] Huang, J.; Hong, J. T.; Hong. S. H. Eur. J. Org. Chem. 2012, 2012, 6630.
[14] Wang, Z. Y.; Chen, G. Q.; Shao, L. X. J. Org. Chem. 2012, 77, 6608.
[15] (a) Berthon-Gelloz, G.; Siegler, M. A.; Spek, A. L.; Tinant, B.; Reek, J. N. H.; Markó, I. E. Dalton Trans. 2010, 39, 1444. (b) Yang, L. G.; Guan, P.; He, P.; Chen, Q.; Cao, C. S.; Peng, Y.; Shi, Z.; Pang, G. S.; Shi, Y. H. Dalton Trans. 2012, 41, 5010. (c) Meiries, S.; Speck, K.; Cordes, D. B.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2013, 32, 330. (d) Organ, M. G.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E. A. B.; O'Brien, C. J.; Valente, C. A. Chem. Eur. J. 2006, 12, 4749.
[16] Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2012, 31, 6947.
/
| 〈 |
|
〉 |