

Chinese Journal of Organic Chemistry >
Recent Progress in Re-Catalyzed Dehydroxylation Reactions
Received date: 2014-09-11
Revised date: 2014-11-11
Online published: 2014-11-20
Supported by
Project supported by the Scientific Research Foundation for Overseas Chinese Scholars of Heilongjiang Province (No. 1154H14), the Department of Education of Heilongjiang Province (No. 1154G53), the Joint Funds of National Natural Science Foundation of China (No. U1362110), and the National Natural Science Foundation of China (Nos. 21322203, 21272238).
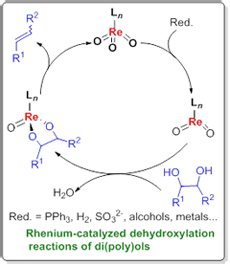
The development of novel effective processes for the conversion of abundant renewable resources to fuels and value-added chemicals has spurred new interest in the discovery of selective chemical transformations of polyols and carbohydrates. These compounds have high oxygen content, which is mostly present in the form of hydroxyl groups. Therefore, partial or complete removal of the hydroxyl groups is of great importance. Herein a brief overview on the development of rhenium-catalyzed dehydroxylation reactions in recent years is given, which are roughly classified into two categories: (1) dehydroxylation reactions of monools for new chemical bond formation and (2) dehydroxylation reactions of diols and polyols for olefin syntheses.
Key words: rhenium catalysis; alcohols; dehydroxylation; reductants; olefins
Mao Guoliang , Jia Bing , Wang Congyang . Recent Progress in Re-Catalyzed Dehydroxylation Reactions[J]. Chinese Journal of Organic Chemistry, 2015 , 35(2) : 284 -293 . DOI: 10.6023/cjoc201409027
[1] (a) Cao, X. H. Chem. Ind. Eng. Prog. 2007, 26, 905 (in Chinese). (曹湘洪, 化工进展, 2007, 26, 905.)
(b) Min, E. CIESC J. 2006, 57, 1739 (in Chinese). (闵恩泽, 化工学报, 2006, 57, 1739.)
(c) Vennestrom, P. N. R.; Osmundsen, C. M.; Christensen, C. H.; Taarning, E. Angew. Chem., Int. Ed. 2011, 50, 10502.
[2] Ragauskas, A. J.; Williams, C. K.; Davison, B. H.; Britovsek, G.; Cairney, J.; Eckert, C. A.; Frederik, W. J. Jr.; Hallett, J. P.; Leak, D. J.; Liotta, C. L.; Mielenz, J. R.; Murphy, R.; Templer, R.; Tschaplinski, T. Science 2006, 311, 484.
[3] Werpy, T.; Peterson, G. Top Value Added Chemicals from Biomass: Vol. 1, U. S. Department of Energy, Washington D. C., 2004.
[4] Corma, A.; Iborra, S.; Velty, A. Chem. Rev. 2007, 107, 2411.
[5] For our work, see: (a) Xia, D.; Wang, Y.; Du, Z.; Zheng, Q.-Y.; Wang, C. Org. Lett. 2012, 14, 588.
(b) Wang, Y.; Zhang, L.; Yang, Y.; Zhang, P.; Du, Z.; Wang, C. J. Am. Chem. Soc. 2013, 135, 18048.
(c) Tang, Q.; Xia, D.; Jin, X.; Zhang, Q.; Sun, X.-Q.; Wang, C. J. Am. Chem. Soc. 2013, 135, 4628.
(d) Tang, H.; Zhou, B.; Huang, X.-R.; Wang, C.; Yao, J.; Chen, H. ACS Catal. 2014, 4, 649. For selected work from other groups, see:
(e) Kuninobu, Y.; Takai, K. Chem. Rev. 2011, 111, 1938.
(f) Jin, H.; Xie, J.; Pan, C.; Zhu, Z.; Cheng, Y.; Zhu, C. ACS Catal. 2013, 3, 2195.
(g) Fukumoto, Y.; Daijo, M.; Chatani, N. J. Am. Chem. Soc. 2012, 134, 8762.
(h) Hua, R.; Tian, X. J. Org. Chem. 2004, 69, 5782.
(i) Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. J. Org. Chem. 2011, 76, 1444.
[6] Zhu, Z.; Espenson, J. H. J. Org. Chem. 1996, 61, 324.
[7] Sherry, B. D.; Radosevich, A. T.; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 6076.
[8] Liu, Y.; Hua, R. M.; Sun, H. B.; Qiu, X. Q. Organometallics 2005, 24, 2819.
[9] Ohri, R. V.; Radosevich, A. T.; Hrovat, K. J.; Musich, C.; Huang, D.; Holman, T. R.; Toste, F. D. Org. Lett. 2005, 7, 2501.
[10] Abdukader, A.; Jin, H.; Cheng, Y.; Zhu, C. Tetrahedron Lett. 2014, 55, 4172.
[11] Xu, Q.; Li, Q. Chin. J. Org. Chem. 2013, 33, 18 (in Chinese). (徐清, 李强, 有机化学, 2013, 33, 18.)
[12] Luzung, M. R.; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 15760.
[13] Kennedy-Smith, J. J.; Young, L. A.; Toste, F. D. Org. Lett. 2004, 6, 1325.
[14] Kuninobu, Y.; Ishii, E.; Takai, K. Angew. Chem., Int. Ed. 2007, 46, 3296.
[15] Nielsen, M. B.; Diederich, F. Synlett 2002, 544.
[16] Kuninobu, Y.; Ueda, H.; Takai, K. Chem. Lett. 2008, 8, 878.
[17] Korstanje, T. J.; Jastrzebski, J. T. B. H.; Klein Gebbink, R. J. M. ChemSusChem 2010, 3, 695.
[18] Korstanje, T. J.; Waard, E. F.; Jastrzeki, J. T. B. H.; Klein Gebbink, R. J. M. ACS Catal. 2012, 2, 2173.
[19] Cook, G. K.; Andrews, M. A. J. Am. Chem. Soc. 1996, 118, 9448.
[20] Raju, S.; Jastrzebski, J. T. B. H.; Robertus, M. L.; Klein Gebbink, R. J. M. ChemSusChem 2013, 6, 1673.
[21] Ziegler, J. E.; Zdilla, M. J.; Evans, A. J.; Abu-Omar, M. M. Inorg. Chem. 2009, 48, 9998.
[22] Denning, A. L.; Dang, H.; Liu, Z. M.; Nicholas, K. M.; Jentoft, F. C. ChemCatChem 2013, 12, 3567.
[23] (a) Holm, R. H.; Donahue, J. P. Polyhedron 1993, 12, 571.
(b) Lee, S. C.; Holm, R. H. Inorg. Chim. Acta 2008, 361, 1166.
[24] Kaksonen, A. H.; Puhakka, J. A. Eng. Life Sci. 2007, 7, 541.
[25] Vkuturi, S.; Chapman, G.; Ahmad, I.; Nicholas, K. M. Inorg. Chem. 2010, 49, 4744.
[26] Ahmad, I.; Chapman, G.; Nicholas, K. M. Organometallics 2011, 30, 2810.
[27] Arceo, E.; Ellman, J. A.; Bergman, R. G. J. Am. Chem. Soc. 2010, 132, 11408.
[28] Yi, J.; Liu, S.; Abu-Omar, M. M. ChemSusChem 2012, 5, 1401.
[29] Shiramizu, M.; Toste, F. D. Angew. Chem., Int. Ed. 2012, 51, 8082.
[30] Shiramizu, M; Toste, F. D. Angew. Chem., Int. Ed. 2013, 52, 12905.
[31] For selected transformations, see: (a) Bellemin-Laponnaz, S. ChemCatChem 2009, 1, 357.
(b) Morrill, C.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 2842.
(c) Morrill, C.; Beutner, G. L.; Grubbs, R. H. J. Org. Chem. 2006, 71, 7813.
(d) Hansen, E. C.; Lee, D. J. Am. Chem. Soc. 2006, 128, 8142.
(e) Herrmann, A. T.; Saito, T.; Stivala, C. E.; Tom, J.; Zakarian, A. J. Am. Chem. Soc. 2010, 132, 5962.
[32] Jacobs, C. B.; Nicholas, K. M. ChemSusChem 2013, 6, 597.
[33] McClain, J. M.; Nicholas, K. M. ACS Catal. 2014, 4, 2109.
/
| 〈 |
|
〉 |