

Chinese Journal of Organic Chemistry >
New Synthesis of Brivudine and Its Analogs
Received date: 2014-10-24
Revised date: 2014-11-22
Online published: 2014-12-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172057, 21272058, 21202040), and the Research Fund for the Doctoral Program of Higher Education (No. 20114104110005).
In this paper, a simple and practical method for the preparation of brivudine (BVDU) and its analog nucleoside derivatives via condensation of the easily obtainable 5-formyl pyrimidine nucleosides with carbon tetrabromide followed by an efficient and stereoselective debromination promoted by diethyl phosphite and triethylamine is presented.
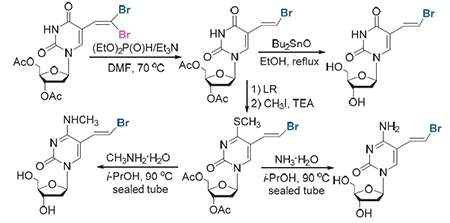
Key words: brivudine; gem-dibromoalkenes; debromination
Li Peiyuan , Zhang Jianrui , Guo Shenghai , Zhang Xinying , Fan Xuesen . New Synthesis of Brivudine and Its Analogs[J]. Chinese Journal of Organic Chemistry, 2015 , 35(4) : 910 -916 . DOI: 10.6023/cjoc201410034
[1] (a) Jordheim, L. P.; Durantel, D.; Zoulim, F.; Dumontet, C. Nat. Rev. Drug Discovery 2013, 12, 447.
(b) Wainberg, M. A. Antiviral Res. 2009, 81, 1.
(c) Jin, Y.-Y.; Xiao, Q.; Jv, Y. Chin. J. Org. Chem. 2009, 29, 44 (in Chinese).
(靳玄烨, 肖强, 巨勇, 有机化学, 2009, 29, 44.)
(d) Menéndez-Arias, L.; álvarez, M.; Pacheco, B. Curr. Opin. Virol. 2014, 8, 1.
[2] (a) Xia, Y.; Liu, Y.; Rocchi, P.; Wang, M.; Fan, Y.; Qu, F.; Iovanna, J. L.; Peng, L. Cancer Lett. 2012, 318, 145.
(b) Wu, J.; Yu, W.; Fu, L.; He, W.; Wang, Y.; Chai, B.; Song, C.; Chang, J. Eur. J. Med. Chem. 2013, 63, 739.
(c) Shirouzu, H.; Morita, H.; Tsukamoto, M. Tetrahedron 2014, 70, 3635.
[3] (a) Wainberg, M. A. Antiviral Res. 2009, 81, 1.
(b) De Clercq, E. Rev. Med. Virol. 2009, 19, 287.
(c) De Clercq, E. J. Med. Chem. 2010, 53, 1438.
(d) De Clercq, E. Antiviral Res. 2010, 85, 19.
[4] Bleackley, R. C.; Jones, A. S.; Walker, R. T. Tetrahedron 1976, 32, 2795.
[5] Ashwell, M.; Jones, A. S.; Kumar, A.; Sayers, J. R.; Walker, R. T.; Sakuma, T.; de Clercq, E. Tetrahedron 1987, 43, 4601.
[6] Wang, C. J.; Xie, F.; Zhang, W. B. Chin. J. Org. Chem. 2008, 28, 503 (in Chinese).
(王春娟, 谢芳, 张万斌, 有机化学, 2008, 28, 503.)
[7] Chelucci, G. Chem. Rev. 2012, 112, 1344 and references cited therein.
[8] (a) Hirao, T.; Masunaga, T.; Ohshiro, Y.; Agawa, T. J. Org. Chem. 1981, 46, 3745.
(b) Abbas, S.; Hayes, C. J.; Worden, S. Tetrahedron Lett. 2000, 41, 3215.
[9] Fan, X. S.; Li, P. Y.; Li, K.; Zhang, X. Y.; Zhao, W. Chin. J. Org. Chem. 2014, 34, 999 (in Chinese).
(范学森, 李培源, 李坤, 张新迎, 赵婉, 有机化学, 2014, 34, 999.)
[10] Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 13, 3769.
[11] (a) Wang, S. M.; Li, J.; Li, H. Y.; Liu, H. M.; Li, W. Chin. J. Org. Chem. 2008, 28, 120 (in Chinese).
(王少敏, 李娟, 李华雨, 刘宏民, 李雯, 有机化学, 2008, 28, 120.)
(b) Li, W.; Liu, H.-M.; You, Q.-D. Acta Chim. Sinica 2003, 61, 1516 (in Chinese).
(李雯, 刘宏民, 尤启东, 化学学报, 2003, 61, 1516.)
/
| 〈 |
|
〉 |