

Chinese Journal of Organic Chemistry >
Progress on Vinylogous Organic Reactions of Allylic Phosphorus Ylides with Carbonyl Compounds
Received date: 2014-07-16
Revised date: 2014-08-11
Online published: 2014-08-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21272119, 21121002), the Research Fund for the Doctoral Program of Higher Education of China (No. 20110031110012), the Fundamental Research Funds for the Central Universities (No. 08143076) and the China Postdoctoral Science Foundation (No. 2014M550484).
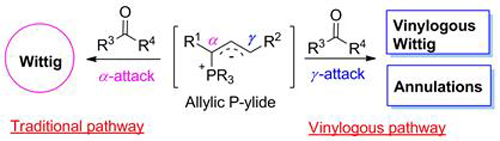
Allylic phosphorus ylides are a class of important and versatile intermediates in synthetic organic chemistry. Due to the delocalization of the carbanion center, an array of vinylogous organic reactions of allylic phosphorus ylides through γ-attack of the carbanion have been achieved in recent years. This mini-review aims to summarize the vinylogous reactivity of allylic phosphorus ylides toward carbonyl compounds, mainly including vinylogous Wittig reactions and various annulation reactions. These reactions broaden the application of allylic phosphorus ylides in organic chemistry, and also provide new synthetic methods for many important organic molecules.
Key words: phosphorus ylide; vinylogy; Wittig reaction; annulation reaction
Xu Silong , He Zhengjie . Progress on Vinylogous Organic Reactions of Allylic Phosphorus Ylides with Carbonyl Compounds[J]. Chinese Journal of Organic Chemistry, 2014 , 34(12) : 2438 -2447 . DOI: 10.6023/cjoc201407027
[1] Fuson, R. C. Chem. Rev. 1935, 16, 1.
[2] Zhang, Y. Univ. Chem. 2002, 17, 55 (in Chinese). (张殷全, 大学化学, 2002, 17, 55.)
[3] Casiraghi, G.; Battistini, L.; Curti, C.; Rassu, G.; Zanardi, F. Chem. Rev. 2011, 111, 3076.
[4] Bur, S. K.; Martin, S. F. Tetrahedron 2001, 57, 3221.
[5] Frank, S. A.; Mergott, D. J.; Roush, W. R. J. Am. Chem. Soc. 2002, 124, 2404.
[6] Kolodiazhnyi, O. I. Phosphorus Ylides: Chemistry and Application in Organic Synthesis, Wiley-VCH, New York, 1999.
[7] Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863.
[8] Howe, R. K. J. Am. Chem. Soc. 1971, 93, 3457.
[9] Schneider, D. F.; Venter, A. C. Synth. Commun. 1999, 29, 1303.
[10] (a) Tamura, R.; Saegusa, K.; Kakihana, M.; Oda, D. J. Org. Chem. 1988, 53, 2723. (b) Tamura, R.; Kato, M.; Saegusa, K.; Kakihana, M.; Oda, D. J. Org. Chem. 1987, 52, 4121. (c) Robiette, R.; Richardson, J.; Aggarwal, V. K.; Harvey, J. N. J. Am. Chem. Soc. 2006, 128, 2394.
[11] Corey, E. J.; Erickson, B. W. J. Org. Chem. 1974, 39, 821.
[12] Boutagy, J.; Thomas, R. Chem. Rev. 1974, 74, 87.
[13] Date, S. M.; Ghosh, S. K. Angew. Chem., Int. Ed. 2007, 46, 386.
[14] (a) Xu, S.; He, Z. Chin. J. Org. Chem. 2012, 32, 1159 (in Chinese). (徐四龙, 贺峥杰, 有机化学, 2012, 32, 1159.) (b) Xu, S.; He, Z. RSC Adv. 2013, 3, 16885. (c) Xu, S.; He, Z. Sci. China, Chem. 2010, 40, 856 (in Chinese). (徐四龙, 贺峥杰, 中国科学: 化学, 2010, 40, 856.)
[15] (a) He, Z.; Tang, X.; He, Z. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 1518. (b) Xu, S.; Chen, R.; He, Z. J. Org. Chem. 2011, 76, 7528.
[16] Khong, S. N.; Tran, Y. S.; Kwon, O. Tetrahedron 2010, 66, 4760.
[17] Liang, Y.; Liu, S.; Yu, Z.-X. Synlett 2009, 905.
[18] (a) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588. (b) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927. (c) Tang, Q.; Tu, A.; Deng, Z.; Hu, M.; Zhong, W. Chin. J. Org. Chem. 2013, 33, 954 (in Chinese). (唐谦, 涂爱平, 邓真真, 胡梦莹, 钟为慧, 有机化学, 2013, 33, 954.) (d) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724. (e) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005. (f) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102. (g) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140. (h) Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520. (i) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035. (j) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535.
[19] Xu, S.; Zhou, L.; Zeng, S.; Ma, R.; Wang, Z.; He, Z. Org. Lett. 2009, 11, 3498.
[20] Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Chem.-Eur. J. 2009, 15, 8698.
[21] Qin, Z.; Ma, R.; Xu, S.; He, Z. Tetrahedron 2013, 69, 10424.
[22] Xiao, H.; Chai, Z.; Yao, R.-S.; Zhao, G. J. Org. Chem. 2013, 78, 9781.
[23] Ma, R.; Xu, S.; Tang, X.; Wu, G.; He, Z. Tetrahedron 2011, 67, 1053.
[24] (a) Li, E.; Huang, Y. Chem.-Eur. J. 2014, 20, 3520. (b) Li, E.; Jia, P.; Liang, L.; Huang, Y. ACS Catal. 2014, 4, 600. (c) Li, E.; Huang, Y. Chem. Commun. 2014, 50, 948. (d) Li, E.; Huang, Y.; Liang, L.; Xie, P. Org. Lett. 2013, 15, 3138. (e) Gicquel, M.; Gomez, C.; Retailleau, P.; Voituriez, A.; Marinetti, A. Org. Lett. 2013, 15, 4002.
[25] (a) Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447. (b) Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659.
[26] Du, Y.; Lu, X.; Zhang, C. Angew. Chem., Int. Ed. 2003, 42, 1035.
[27] Zhou, R.; Wang, C.; Song, H.; He, Z. Org. Lett. 2010, 12, 976.
[28] Wang, T.; Shen, L.-T.; Ye, S. Synthesis 2011, 3359.
[29] Chen, Z.; Zhang, J. Chem.-Asian J. 2010, 5, 1542.
[30] (a) Tian, J.; Zhou, R.; Sun, H.; Song, H.; He, Z. J. Org. Chem. 2011, 76, 2374. (b) Zhou, R.; Duan, C.; Yang, C.; He, Z. Chem.-Asian J. 2014, 9, 1183. (c) Tian, J.; Sun, H.; Zhou, R.; He, Z. Chin. J. Chem. 2013, 31, 1348.
[31] Xie, P.; Li, E.; Zheng, J.; Li, X.; Huang, Y.; Chen, R. Adv. Synth. Catal. 2013, 355, 161.
[32] Xie, P.; Huang, Y.; Chen, R. Chem.-Eur. J. 2012, 18, 7362.
[33] (a) Shi, M.; Hu, F.-L.; Wei, Y. Chem. Commun. 2014, 50, 8912. (b) Zhang, X. N.; Deng, H. P.; Huang, L.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 8664.
[34] (a) Bohlmann, F.; Zdero, C. Chem. Ber. 1973, 106, 3779. (b) Büchi, G.; Wüest, H. Helv. Chim. Acta 1971, 54, 1767. (c) Padwa, A.; Brodsky, L. J. Org. Chem. 1974, 39, 1318.
[35] Dauben, W. G.; Hart, D. J.; Ipaktschi, J.; Kozikowski, A. P. Tetrahedron Lett. 1973, 14, 4425.
[36] Martin, S. F.; Desai, S. R. J. Org. Chem. 1977, 42, 1664.
[37] Dauben, W. G.; Ipaktschi, J. J. Am. Chem. Soc. 1973, 95, 5088.
[38] Wang, Q.; Sun, X.; Zheng, J.; Tang, Y. Chin. J. Chem. 2010, 28, 1618.
[39] (a) Ye, L.-W.; Han, X.; Sun, X.-L.; Tang, Y. Tetrahedron 2008, 64, 8149. (b) Shu, Z.-C.; Zhu, J.-B.; Liao, S.; Sun, X.-L.; Tang, Y. Tetrahedron 2013, 69, 284.
[40] Liu, S.; Chen, W.; Luo, J.; Yu, Y. Chem. Commun. 2014, 50, 8539.
[41] Ye, L.-W.; Wang, S.-B.; Wang, Q.-G.; Sun, X.-L.; Tang, Y.; Zhou, Y.-G. Chem. Commun. 2009, 3092.
[42] Yadav, J. S.; Srinivas, D. Tetrahedron Lett. 1997, 38, 7789.
[43] Bennani, Y. L.; Boehm, M. F. J. Org. Chem. 1995, 60, 1195.
[44] (a) Himeda, Y.; Hatanaka, M.; Ueda, I. J. Chem. Soc., Chem. Commun. 1995, 449. (b) Himeda, Y.; Yamataka, H.; Ueda, I.; Hatanaka, M. J. Org. Chem. 1997, 62, 6529.
[45] Himeda, Y.; Ueda, I.; Hatanaka, M. Chem. Lett. 1996, 25, 71.
[46] Hatanaka, M.; Himeda, Y.; Ueda, I. J. Chem. Soc., Perkin Trans. 1 1993, 2269.
[47] (a) Hatanaka, M.; Himeda, Y.; Ueda, I. J. Chem. Soc., Chem. Commun. 1990, 526. (b) Hatanaka, M.; Himeda, Y.; Imashiro, R.; Tanaka, Y.; Ueda, I. J. Org. Chem. 1994, 59, 111. (c) Hatanaka, M.; Himeda, Y.; Ueda, I. Tetrahedron Lett. 1991, 32, 4521.
[48] Wang, P.; Liao, S.; Zhu, J.-B.; Tang, Y. Chem. Commun. 2014, 50, 808.
[49] Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13 (in Chinese). (欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
/
| 〈 |
|
〉 |