

Chinese Journal of Organic Chemistry >
Optimization of the Synthetic Process of Antineoplastic Drug Dacarbazine
Received date: 2014-10-18
Revised date: 2014-12-19
Online published: 2015-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No.81172982).
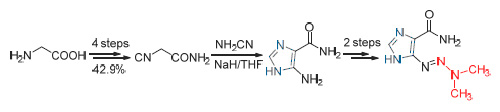
Dacarbazine has been prepared started from glycine, which was carried on firstly through a four-step process including esterification, formylation, dehydration, amidation to give 2-isocyanoacetamide with a total yield of 42.9%. 2-Isocyanoacetamide was then cyclized with cyanamide under basic condition to afford the important intermediate 5-amino-1H-imidazole-4-carboxamide with yield of 68.2%. This intermediate undergoing diazotization and following coupling reaction with dimethylamine led to the target compound. The overall yield was 22.3%. All the intermediates and the target products dacarbazine were confirmed by 1H NMR, 13C NMR and ESI-MS. The current process is economic efficient charac-terized by applied cheap materials, mild conditions, and good overall yield.
Key words: dacarbazine; process optimization; drug synthesis; anticancer
Xiao Shengwei , Liu Zhijun , Liu Yang , Chen Heru . Optimization of the Synthetic Process of Antineoplastic Drug Dacarbazine[J]. Chinese Journal of Organic Chemistry, 2015 , 35(5) : 1161 -1165 . DOI: 10.6023/cjoc201410026
[1] Wexler, P. In Encyclopedia of Toxicology, 3rd ed., Ed.: Andrea, M., Manchester, 2014, pp. 1132~1134.
[2] Zheng, M.; Sun, G. J. J. Pract. Oncol. 1998, 13(6), 328 (in Chinese). (郑敏, 孙国君, 实用肿瘤学杂志, 1998, 13(6), 328.)
[3] Pretto, F.; Elia, G.; Castioni, N.; Neri, D. Cancer Immunol. Immunother. 2014, 63(9), 901.
[4] Alexandrina, C.; Cornea, F.; Ionescu, C. D. RO 80163, 1983 [Chem. Abstr. 1984, 101, 151849].
[5] Jain, M. L.; Tsao, Y. P.; Ho, N. L.; Cheng, J. W. J. Org. Chem. 2001, 66, 6472.
[6] Mitsuru, T.; Masami, H.; Yoshikazu, I. JP 544941, 2004 [Chem. Abstr. 2004, 354906].
[7] Chaudhary, S.; Singh, K. R. Asian J. Pharm. Clin. Res. 2012, 5(4), 196.
[8] Panella, L.; Aleixandre, A. M.; Kruidhof, G. J.; Robertus, J. J. Org. Chem. 2006, 71, 2026.
[9] Tomasz, G.; Robert, H.; Scott, H. US 13983747, 2013 [Chem. Abstr. 2012, 157, 383004].
[10] Elders, N.; Ruijter, E.; Frans, J. J. Chem. Eur. J. 2009, 15, 6096.
[11] Elders, N.; Ruijter, E.; Frans, J. J. Chem. Eur. J. 2008, 14, 4961.
[12] Murakami, T.; Otsuka, M.; Ohno, M. Tetrahedron Lett. 1982, 23(45), 4729.
[13] Saladino, R.; Crestini, C.; Ciambecchini, U.; Ciciriello, F.; Cos-tanzo, G.; Di Mauro, E. ChemBioChem 2004, 5, 1558.
/
| 〈 |
|
〉 |