

Chinese Journal of Organic Chemistry >
Synthesis and Antifungal Activity of Novel 3-[(5-Benzylthio- 1,3,4-oxadiazol-2-yl)methyl]benzo[d]thiazol- (oxazol)-2(3H)-ones
Received date: 2014-10-31
Revised date: 2014-12-14
Online published: 2015-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No.30900959) and Public Project of Zhejiang Province (No.2014C31127).
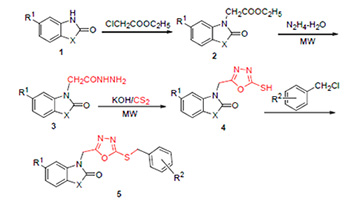
In order to find novel biologically active pesticide lead compounds, twenty-one novel 3-[(5-benzylthio-1,3,4- oxadiazol-2-yl)methyl]benzo[d]thiazol(oxazol)-2(3H)-ones were synthesized by 2-benzothiazolinone/benzoxazolone as starting materials via substitution, hydrazine, cyclization and the last benzylation reaction. The structures of the title compounds were characterized by 1H NMR, IR, EI-MS and elemental analysis. The preliminary bioassay results indicated that most of them showed moderate inhibition activity against Colletotrichum orbiculare, Botrytis cinerea and Rhizoctonia solani at 50 mg/L, and the inhibition rate of compound 5b against Botrytis cinerea and Rhizoctonia solani reached above 85%.
Ruan Lingli , Fan Renjie , Liu Xinghai , Chen Jie , Weng Jianquan . Synthesis and Antifungal Activity of Novel 3-[(5-Benzylthio- 1,3,4-oxadiazol-2-yl)methyl]benzo[d]thiazol- (oxazol)-2(3H)-ones[J]. Chinese Journal of Organic Chemistry, 2015 , 35(5) : 1166 -1172 . DOI: 10.6023/cjoc201410041
[1] Strange, R. N.; Scott, P. R. Annu. Rev. Phytopathol. 2005, 43, 83.
[2] Li, B.; Liu, B.-P.; Yu, R.-R.; Lou, M.-M.; Wang, Y.-L.; Xie, G.-L.; Li, H.-Y.; Sun, G.-C. World J. Microbiol. Biotechnol. 2011, 27, 2305.
[3] Groth, D. E. Crop Prot. 2008, 27, 1125.
[4] Mahran, M. A.; Einassry, S. M.; Allam, S. R. Pharmazie 2003, 58, 527.
[5] Sidoova, E.; Loos, D.; Bujdakova, H. Molecules 1997, 2, 36.
[6] Wang, W.; Zhang, G.-P.; Song, B.-A.; Wang, H.; Jin, L.-H.; Hu, D.-Y.; Yang, S. Chin. J. Org. Chem. 2007, 27, 279 (in Chinese). (王伟, 张国平, 宋宝安, 汪华, 金林红, 胡德禹, 杨松, 有机化学, 2007, 27, 279.)
[7] Ganzer, M.; Dorfmeister, G.; Franke, W.; Johann, G.; Rees, R. DE 4117508, 1992 [Chem. Abstr. 1993, 118, 101946].
[8] David, P. C.; Roy, V. E.; Roy, T. H. J. Agric. Food Chem. 1981, 29, 640.
[9] Gewehr, M.; Dietz, J.; Grote, T.; Haden, E. WO 2009147205, 2009 [Chem. Abstr. 2010, 152, 113128].
[10] Michel, S. US 3922281, 1975 [Chem. Abstr. 1975, 82, 170877].
[11] Toyabe, K.; Shimizu, K.; Hirata, M.; Ikeda, T. JP 0217187, 1990 [Chem. Abstr. 1990, 113, 59158].
[12] Kraus, A.; Ishikawa, K. WO 2004095930, 2004 [Chem. Abstr. 2004, 141, 390414].
[13] Sidoova, E.; Loos, D.; Bujdakova, H.; Kallova, J. Molecules 1997, 2, 36.
[14] Koci, J.; Klimesova, V.; Waisser, K. Bioorg. Med. Chem. Lett. 2002, 12, 22.
[15] Hutchinson, I.; Chua, M. S.; Browne, H. L. J. Med. Chem. 2001, 4, 9.
[16] Huang, Q.-C.; Qian, X.-H.; Song, G.-H.; Cao, S. Pest Manage. Sci. 2003, 59, 933.
[17] Mo, Q.-J.; Duan, W.-G.; Li, X.-R.; Huang, D.-P.; Luo, D.-C. Chin. J. Org. Chem. 2011, 31, 1114 (in Chinese). (莫启进, 段文贵, 李行任, 黄丹平, 罗德城, 有机化学, 2011, 31, 1114.)
[18] Omar, F.-A.; Mahfouz, N.-M.; Rahman, M.-A. Eur. J. Med. Chem. 1996, 31, 819.
[19] Li, C.; Tan, Z.-L.; Li, X.-W.; Zhang, X. Chin. J. Org. Chem. 2005, 25, 5 (in Chinese). (李超, 覃章兰, 李秀文, 张欣, 有机化学, 2005, 25, 5.)
[20] Li, Q.-Z.; Song, B.-A.; Chen, J. Agrochemicals 2005, 44, 12 (in Chinese). (李黔柱, 宋宝安, 陈江, 农药, 2005, 44, 12.)
[21] Weng, J.-Q.; Liu, X.-H.; Huang, H.; Tan, C.-X.; Chen, J. Molecules 2012, 17, 989.
[22] Che, C.; Mao, S.-F.; Tan, Z.-H. Chin. Appl. Chem. 2002, 19, 8 (in Chinese). (车超, 毛淑芬, 覃兆海, 应用化学, 2002, 19, 8.)
[23] Elwahy, A. H.; Abbas, A. A. Tetrahedron 2000, 56, 885.
[24] Chen, H.-S.; Li, Z.-M.; Han, Y.- F. J. Agric. Food Chem. 2000, 48, 5312.
[25] Bao, X.-P.; Lin, X.-F.; Zhang, F.; Zhou, L.-B. Chin. J. Org. Chem. 2013, 33, 995 (in Chinese). (鲍小平, 林选福, 张峰, 邹林波, 有机化学, 2013, 33, 995.)
[26] Zhong, B.; Fan, Z.-J.; Li, Z.-M. Chin. Appl. Chem. 2003, 20, 7 (in Chinese). (钟斌, 范志金, 李正名, 应用化学, 2003, 20, 7.)
[27] De Sousa, G.; Nawaz, A.; Cravedi, J. P.; Rahmani, R. Toxicol. Sci. 2014, 141, 1.
[28] Chen, W.-B.; Jin, G.-Y. Acta Chim. Sinica 2001, 22, 1147 (in Chinese). (陈文彬, 金桂玉, 化学学报, 2001, 22, 1147.)
[29] Wang, L.; Asimakopoulos, A. G.; Moon, H. B.; Nakata, H.; Kannan, K. Environ. Sci. Technol. 2013, 47, 9.
[30] Amico, D. J.; Bollinger, F. G. Heterocycl. Chem. 1988, 25, 1183.
[31] Li, Y.-J.; Li, C.-Y.; Sun, S.-Q.; Zhou, X.-X. Acta Chim. Sinica 2012, 70, 2 (in Chinese). (李英俊, 李春燕, 孙淑琴, 周晓霞, 化学学报, 2012, 70, 2.)
/
| 〈 |
|
〉 |