

Chinese Journal of Organic Chemistry >
Total Synthesis of Longistylin A, Longistylin C and Cajanotone
Received date: 2014-10-17
Revised date: 2014-12-05
Online published: 2015-01-09
Supported by
Project supported by the Program for New Century Excellent Talents in University (No.NCET-12-0656), the Innovation Ability Promoting Program in University of Guizhou Province (No.2013-04), the Natural Science Fundation for Youth in Universities of Guizhou Province (No.2014-300).
Natural products longistylin A, longistylin C and cajanotone belong to stilbenes family and exhibit varieties of bioactivities. Using Horner-Wadsworth-Emmons (HWE) and 1,3-sigma rearrangement reactions as key steps, longistylin A and longistylin C have been successfully synthesized in six steps from 3,5-dimethoxy benzoic acid. Besides, using Barbier and 1,3-sigma rearrangement reactions as key steps, cajanotone has been first prepared in eight steps from 3,5-dihydroxy benzoic acid.
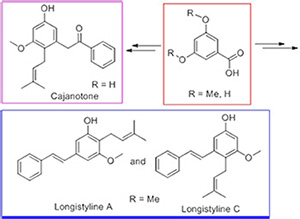
Key words: longistylin A; longistylin C; cajanotone; total synthesis
Chen Wenzhang , Wang Cong , Fan Lingling , Wang Jianta , Yue Weizhou , Tang Lei . Total Synthesis of Longistylin A, Longistylin C and Cajanotone[J]. Chinese Journal of Organic Chemistry, 2015 , 35(5) : 1146 -1151 . DOI: 10.6023/cjoc201410024
[1] Hopp, D. C.; Inman, W. D. US 20020058701, 2002 [Chem. Abstr. 2002, 136, 205396].
[2] Zhang, G. L.; Hong, T.; Zhang, N. L.; Yu, B.; Qiu, S. X.; Lu, Y. Chemistry 2012, 75, 565 (in Chinese). (张贵林, 洪挺, 张嫩玲, 余勃, 邱声祥, 陆豫, 化学通报, 2012, 75, 565.)
[3] Luo, Q. F.; Sun, L.; Si, J. Y.; Chen, D. H.; Du, G. H. Acta Pharm. Sin. 2008, 43, 145 (in Chinese). (骆庆峰, 孙兰, 斯建勇, 陈迪华, 杜冠华, 药学学报, 2008, 43, 145.)
[4] Ashidi, J. S.; Houghton, P. J.; Hylands, P. J.; Efferth, T. J. Ethnopharmacol. 2010, 128, 501.
[5] Jiang, B. P.; Yang, R. W.; Liu, X. M.; Liu, Y. M.; Chang, Q.; Si, J. Y.; Pan, R. L. Acta Pharm. Sin. 2012, 47, 600 (in Chinese). (姜保平, 杨瑞武, 刘新民, 刘亚旻, 常琪, 斯建勇, 潘瑞乐, 药学学报, 2012, 47, 600.)
[6] Zhang, N. L.; Zhu, Y. H.; Huang, R. M.; Fu, M. Q.; Su, Z. W.; Cai, J. Z.; Hu, Y. J.; Qiu S. X. Z. Naturforsch. 2012, 67b, 1314.
[7] Ji, X.-Y. Ph.D. Dissertation, Chinese Academy of Science Pe-king Union Medical College, Peking, 2011 (in Chinese). (季兴跃, 博士论文, 北京协和医学院, 北京, 2011.)
[8] Davis, M. C.; Groshens, T. J. Tetrahedron Lett. 2012, 53, 3521.
[9] Xu, Z.-H, Pan, S.; Huang, Y.-G. Chin. J. Org. Chem. 2014, 34, 1391 (in Chinese). (徐之涵, 潘燊, 黄焰根, 有机化学, 2014, 34, 1391.)
[10] Heynekamp, J. J.; Weber, W. M.; Hunsaker, L.; Gonzales, A. M.; Orlando, R. A.; Deck, L. M.; Vander Jagt, D. L. J. Med. Chem. 2006, 49, 7182.
[11] Xia, L.; Lee, Y. R. Synlett 2008, 1643.
[12] Bachelor, F. W.; Loman, A. A.; Snowdon, L. R. Can. J. Chem. 1970, 48, 1554.
[13] Koolaji, N.; Abu-Mellal, A.; Tran, V. H.; Duke, R. K.; Duke, C. C. Eur. J. Med. Chem. 2013, 63, 415.
[14] Meltzer, P. C.; Dalzell, H. C.; Razdan, R. K. J. Chem. Soc, Perkin Trans. 1 1981, 2815.
[15] Zhu, J. P.; Beugelmans, R.; Bourdet, S.; Chastanet, J.; Roussi, G. J. Org. Chem. 1995, 60, 6389.
[16] Bieber, L. W.; Storch, E. C.; Malvestiti, I.; Silva, M. F. D. Tetrahedron Lett. 1988, 39, 9393.
[17] Huang, J. H.; Foyle, D.; Lin, X. R.; Yang, J. J. Org. Chem. 2013, 78, 9166.
[18] Chen, W. Z.; Fan, L. L.; Xiao, H. T.; Zhou, Y.; Xiao, W.; Wang, J. T.; Tang, L. Chin. Chem. Lett. 2014, 25, 749.
/
| 〈 |
|
〉 |