

Chinese Journal of Organic Chemistry >
Synthesis and Fluorescent Properties of Water-Soluble 1,8-Naphthalimide Dendron
Received date: 2014-11-06
Revised date: 2014-12-15
Online published: 2015-01-14
Supported by
Project supported by the National Natural Science Foundation of China (No.61178057).
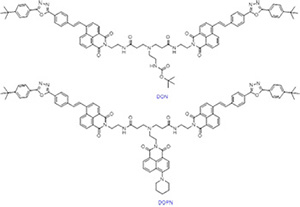
Two novel compounds, water-soluble 1,8-naphthalimide dendrons tert-butoxycarbonyl-{N,N-bis{3,5-bis{2-(4- tert-butylphenyl)-5-[1,3,4]-oxadiazolyl}-styryl-1,8-naphathlimide}-ethylaminocarbonylethyl}ethylamino}-1,8-naphthalimide (DON) and 4-piperidyl-9-ethylamino-{N,N-bis{3,5-bis{2-(4-tert-butylphenyl)-5-[1,3,4]-oxadiazolyl}-styryl-1,8-naphathli- mide}-ethylaminocarbonylethyl}ethylamino}-1,8-naphthalimide (DOPN) were synthesized and characterized by 1H NMR, 13C NMR and HRMS techniques. Fluorescence spectra of DON and DOPN in solid, tetrahydrofuran and aqueous were measured. The results demonstrated that DON and DOPN performed orange fluorescence in the solid state and the maximum emission wavelength were 566 and 561 nm, respectively. Blue-green fluorescence was observed in tetrahydrofuran (THF) solution. The maximum absorption wavelengths were 399 and 395 nm, the maximum emission wavelengths were 489 and 492 nm, and the quantum yields were 28.2% and 20.4%, respectively. Besides, the maximum absorption wavelengths were 406 and 408 nm, and the maximum emission wavelength were 563 and 552 nm in aqueous. Two water-soluble 1,8-naphthalimide dendrons were applied for fluorescence imaging in Hela living cells. In addition, metal ion probe behavior of DON was detected. And DON exhibitted a selectivity for tin ion. With the incresing of concentration of tin ion, fluorescence intensity of DON was enhancing. The number of binding sites between tin ion and DON was 2. In a word, two water-soluble 1,8-naphthalimide dendrons, can be used as a flourescent probe for detection of tin, and can be further applied in living cell imaging.
Sun Jingfu , Qian Ying . Synthesis and Fluorescent Properties of Water-Soluble 1,8-Naphthalimide Dendron[J]. Chinese Journal of Organic Chemistry, 2015 , 35(5) : 1104 -1111 . DOI: 10.6023/cjoc201411008
[1] Duke, R. M.; Gunnlaugsson, T. Tetrahedron Lett. 2007, 48, 8043.
[2] Zhu, L.; Yuan, Z.; J. Tyler, S.; Kesavapillai, S. RSC Adv. 2014, 4, 20398.
[3] Xu, Z.-C.; Juyoung, Y.; David, R. S. Chem. Soc. Rev. 2010, 39, 1996
[4] Xuan-Anh, T.; Victor, A.; Paolo, B.; Bernadette, T. S. B.; Karsten, H. Biosens. Bioelectron. 2015, 64, 359.
[5] Gan, J.-A.; Song, Q.-L; Hou, X.-Y.; Chen, K.-C.; Tian, H. J. Photochem. Photobiol. A 2004, 162, 399.
[6] Jin, P.-W.; Jiao, C.-H.; Guo, Z.-Q.; He, Y.; Zhu, S.-Q.; Tian, H.; Zhu, W.-H. Chem. Sci. 2014, 5, 4012
[7] Liu, X.-L; Du, X.-J.; Dai, C.-G; Song, Q.-H. J. Org. Chem. 2014, 79, 9481.
[8] Liu, Y.-B.; Liu, Y.-W; Liu, W.; Liang, S.-C. Spectrochim. Acta, A 2015, 137, 509
[9] Liu, Y.; Sun, K.-X.; Yao, W.-J.; Liang, N.; Mu, H.-J.; Liang, R.-C.; Yao, C. Chem. J. Chin. Univ. 2010, 31, 800 (in Chinese). (刘毅, 孙考祥, 姚文军, 梁娜, 慕宏杰, 梁荣才, 姚晨, 高等学校化学学报, 2010, 31, 800.)
[10] Zeng, Y.; Li, Y.-Y.; Yuan, Z.; Li, Y. Acta Chim. Sinica 2009, 67, 2714 (in Chinese). (曾毅, 李迎迎, 袁钊, 李嫕, 化学学报, 2009, 67, 2714.)
[11] Liu, B.; Tian, H. Chem. Commun. 2005, 3156.
[12] Khalid, A. A.; Nikolai, I. G.; Samy, A. E.-D.; Layla, A. T.; Vladimir, B. B. J. Lumin. 2015, 158, 50.
[13] Stanislava, Y.; Ivo, G.; Stanimir, S.; Ivan, P. J. Photochem. Photobiol. A 2014, 283, 1.
[14] Liu, D.; Qi, J.; Liu, X.-Y.; Cui, Z.-G.; Chang, H.-X.; Chen, J.-T.; Yang, G.-M. Sens. Actuators, B 2014, 204, 655
[15] Xu, L.; Xu, Y.-F.; Zhu, W.-P.; Yang, C.-M.; Han, L.; Qian, X.-H. Dalton Trans. 2012, 41, 7212
[16] Sun, Y.; Liang, X.-H.; Fan, J.; Han, Q. J. Lumin. 2013, 141, 93
[17] Sun, Y.; Liu, Z.; Liang, X.-H; Fan, J.; Han, Q. Spectrochim. Acta, A 2013, 108, 8.
[18] Li, Y.-H.; Wu, Y.-Q.; Chang, J.; Chen, M.; Liu, R.; Li, Y.-F. Chem. Commun. 2013, 49, 11335.
[19] Sanjoy, M.; Pakkirisamy, T. Chem. Commun. 2013, 49, 7292.
[20] Zhang, G.-F.; Matthew, P. A.; Gong, W.-L.; Li, C.; Zhu, M.-Q. Chem. Commun. 2012, 48, 7711.
[21] Luo, X.-Y.; Qian, Y. Chin. J. Org. Chem. 2013, 33, 2423 (in Chinese). (罗晓燕, 钱鹰, 有机化学, 2013, 33, 2423.)
[22] Qian, Y.; Meng, K.; Lu, C.-G.; Lin, B.-P.; Huang, W.; Cui, Y.-P. Dyes Pigm. 2009, 80, 174
[23] Qian, Y.; Lv, X.-M.; Zhou, Z.-Q.; Cui, Y.-P. Chem. J. Chin. Univ. 2011, 32, 2441 (in Chinese). (钱鹰, 闾新明, 周志强, 崔一平, 高等学校化学学报, 2011, 32, 2441.)
[24] Lv, X.-M.; Qian, Y. Chin. J. Org. Chem. 2011, 31, 82 (in Chinese). (闾新明, 钱鹰, 有机化学, 2011, 31, 82.)
[25] Wang, B.-B.; Qian, Y. Chin. J. Org. Chem. 2014, 34, 210 (in Chinese). (王彬彬, 钱鹰, 有机化学, 2014, 34, 210.)
[26] Guan, C.-F.; Qian, Y. Chin. J. Org. Chem. 2014, 34, 537 (in Chinese). (管成飞, 钱鹰, 有机化学, 2014, 34, 537.)
[27] Yang, H.; Qi, Q.; Wang, B.-B.; Qian, Y. Chem. J. Chin. Univ. 2013, 34, 1880 (in Chinese). (杨铧, 齐齐, 王彬彬, 钱鹰, 高等学校化学学报, 2013, 34, 1880.)
[28] Wang, B.-B.; Qian, Y. New J. Chem. 2013, 37, 1402.
[29] Meka, N.; Temesgen, T.; Siraj, K. J. Biol. Chem. Res. 2014, 31, 138.
[30] Peng, X.-J.; Du, J.-J.; Fan, J.-L.; Wang, J.-Y.; Wu, U.-K.; Zhao, J.-Z.; Sun, S.-G.; Xu, T. J. Am. Chem. Soc. 2007, 129, 1500.
[31] Zhu, X.-Q.; Qian, Y. Chin. J. Org. Chem. 2009, 29, 1975 (in Chinese). (朱晓勤, 钱鹰, 有机化学, 2009, 29, 1975.)
[32] Meng, K.; Qian Y. Chin. J. Org. Chem. 2009, 29, 71 (in Chinese). (孟康, 钱鹰, 有机化学, 2009, 29, 71.)
[33] Mark, D.M.; Ivo, G.; Paula, B. Dyes Pigm. 2009, 81, 180.
/
| 〈 |
|
〉 |