

Chinese Journal of Organic Chemistry >
Transition-Metal-Catalyzed C—H Alkynylation
Received date: 2014-12-29
Revised date: 2015-01-26
Online published: 2015-01-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21472211).
Alkynyl compounds are paramount skeletons in pharmaceuticals and natural products, and great importance has been attached to its synthesis by organic chemists. Recently, transition-metal-catalyzed C—H alkynylation has attracted tremendous interest as a valuable tool for construction of alkynyl compounds. This review gives a summary of progress having been made in the field of the C(sp2)—H and C(sp3)—H alkynylation over the last ten years.
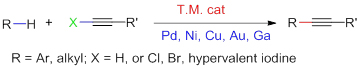
Key words: alkynylation; C—H activation; alkyne; cross dehydrogen coupling
Wang Mingming , Wang Zixiao , Shang Ming , Dai Huixiong . Transition-Metal-Catalyzed C—H Alkynylation[J]. Chinese Journal of Organic Chemistry, 2015 , 35(3) : 570 -577 . DOI: 10.6023/cjoc201412048
[1] Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 16, 4467.
[2] Dudnik, A. S.; Gevorgyan, V. Angew. Chem., Int. Ed. 2010, 49, 2096.
[3] Kobayashi, K.; Arisawa, M.; Yamaguchi, M. J. Am. Chem. Soc. 2002, 124, 8528.
[4] Amemiya, R.; Fujii, A.; Yamaguchi, M. Tetrahedron Lett. 2004, 45, 4333.
[5] Tobisu, M.; Ano, Y.; Chatani, N. Org. Lett. 2009, 11, 3250.
[6] Kalinin, V. K.; Pashchenko, D. N.; She, F. M.; Mendeleev. Commun. 1992, 2, 60.
[7] Trofimov, B. A.; Stepanova, Z. V.; Sobenina, L. N.; Mikhaleva, A. B. I.; Ushakov, I. A. Tetrahedron Lett. 2004, 45, 6513.
[8] (a) Sobenina, L. N.; Demenev, A. P.; Mikhaleva, A. b. I.; Ushakov, I. A.; Vasil'tsov, A. M.; Ivanov, A. V.; Trofimov, B. A. Tetrahedron Lett. 2006, 47, 7139. (b) Gu, Y.; Wang, X.-M. Tetrahedron Lett. 2009, 50, 763.
[9] Seregin, I. V.; Gevorgyan, V. Chem. Soc. Rev. 2007, 36, 1173.
[10] (a) Matsuyama, N.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2009, 11, 4156. (b) Besselievre, F.; Piguel, S. Angew. Chem., Int. Ed. 2009, 48, 9553. (c) Kim, S. H.; Chang, S. Org. Lett. 2010, 12, 1868.
[11] Kawano, T.; Matsuyama, N.; Hirano, K.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2010, 75, 1764.
[12] Chatani, N.; Tobisu, M.; Ano, Y. Synlett 2012, 23, 2763.
[13] Ano, Y.; Tobisu, M.; Chatani, N. Org. Lett. 2012, 14, 354.
[14] Xu, Y.-H.; Zhang, Q.-C.; He, T.; Meng, F.-F.; Loh, T.-P. Adv. Synth. Catal. 2014, 356, 1539.
[15] Brand, J. P.; Charpentier, J.; Waser, J. Angew. Chem., Int. Ed. 2009, 48, 9346.
[16] Tolnai, G. L.; Ganss, S.; Brand, J. P.; Waser, J. Org. Lett. 2013, 15, 112.
[17] Brand, J. P.; Waser, J. Angew. Chem., Int. Ed. 2010, 49, 7304.
[18] Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem., Int. Ed. 2009, 48, 4332.
[19] Brand, J. P.; Waser, J. Org. Lett. 2012, 14, 744.
[20] Li, Y.; Waser, J. Beilstein J. Org. Chem. 2013, 9, 1763.
[21] Feng, C.; Loh, T. P. Angew. Chem., Int. Ed. 2014, 53, 2722.
[22] (a) Xie, F.; Qi, Z.; Yu, S.; Li, X. J. Am. Chem. Soc. 2014, 136, 4780. (b) Zhang, X.; Qi, Z.; Gao, J.; Li, X. Org. Biomol. Chem. 2014, 12, 9329.
[23] Wu, Y.; Yang, Y.; Zhou, B.; Li, Y. J. Org. Chem. 2015, 80, 1946.
[24] Collins, K. D.; Lied, F.; Glorius, F. Chem. Commun. 2014, 50, 4459.
[25] Feng, C.; Feng, D.; Loh, T.-P. Chem. Commun. 2014, 50, 9865.
[26] Feng, C.; Feng, D.; Luo, Y.; Loh, T. P. Org. Lett. 2014, 16, 5956.
[27] Matsuyama, N.; Kitahara, M.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2010, 12, 2358.
[28] Wei, Y.; Zhao, H.; Kan, J.; Su, W.; Hong, M. J. Am. Chem. Soc. 2010, 132, 2522.
[29] Haro, T.; Nevado, C. J. Am. Chem. Soc. 2010, 132, 1512.
[30] Kawano, T.; Matsuyama, N.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2010, 75, 1764.
[31] Yang, L.; Zhao, L.; Li, C.-J. Chem. Commun. 2010, 46, 4184.
[32] Kim, S. H.; Yoon, J.; Chang, S. Org. Lett. 2011, 13, 1474.
[33] Jie, X.; Shang, Y.; Hu, P.; Su, W. Angew. Chem., Int. Ed. 2013, 52, 3630.
[34] Weng, Y.; Cheng, B.; He, C.; Lei, A. Angew. Chem., Int. Ed. 2012, 51, 9547.
[35] Kim, S. H.; Park, S. H.; Chang, S. Tetrahedron 2012, 68, 5162.
[36] Shang, M.; Wang, H.-L.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 11590.
[37] Liu, Y.-J.; Liu, Y.-H.; Yin, X.-S.; Gu, W.-J.; Shi, B.-F. Chem. Eur. J. 2015, 21, 205.
[38] Ano, Y.; Tobisu, M.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 12984.
[39] Al-Amin, M.; Arisawa, M.; Shuto, S.; Ano, Y.; Tobisu, M.; Chatani, N. Adv. Synth. Catal. 2014, 356, 1631.
[40] He, J.; Wasa, M.; Chan, K. S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 3387.
/
| 〈 |
|
〉 |