

Chinese Journal of Organic Chemistry >
Synthesis of Calix[4]proline Derivatives and Their Chiral Recognition for Enantiomers of Mandelic Acid
Received date: 2015-02-10
Revised date: 2015-03-02
Online published: 2015-03-19
Supported by
Project supported by the NSFC (No. 21002009), Major Program for the Natural Science Research of Jiangsu Colleges and Universities (Nos. 12KJA150002, 14KJA150002), Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD), and Qing Lan Project of Jiangsu Province.
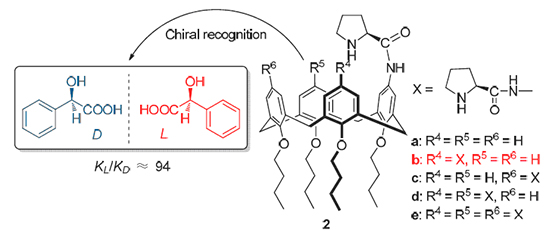
Five chiral calix[4]proline derivatives were designed and synthesized from aminocalix[4]arenes, and their structures were characterized by 1H NMR, 13C NMR, IR, ESI-MS and elemental analysis. The chiral recognition ability for enantiomers of mandelic acid was investigated by NMR techniques, and the results showed that 5,11-diprolinamides substituted calix[4]arene 2b could well and selectively recognize the two enantiomers of mandelic acid (KL/KD≈94). A possible recognition mechanism was proposed, which was constructed by cooperative multi-supramolecular interactions including intermolecular acid-base, hydrogen bond and π···π stacking interactions.
Key words: calix[4]arene; L-proline; mandelic acid; chiral recognition; NMR
Li Zhengyi , Zhou Kun , Lai Yuan , Sun Xiaoqiang , Wang Leyong . Synthesis of Calix[4]proline Derivatives and Their Chiral Recognition for Enantiomers of Mandelic Acid[J]. Chinese Journal of Organic Chemistry, 2015 , 35(7) : 1531 -1536 . DOI: 10.6023/cjoc201502018
[1] Zheng, B.; Wang, F.; Dong, S. Y.; Huang, F. H. Chem. Soc. Rev. 2012, 41, 1621.
[2] (a) Han, C. P.; Hou, X.; Zhang, H. C.; Guo, W.; Li, H. B.; Jiang, L. J. Am. Chem. Soc. 2011, 133, 7644.(b) Jin, Q. X.; Li, J.; Li, X. G.; Zhang, L.; Fang, S. M.; Liu, M. H. Prog. Chem. 2014, 26, 919 (in Chinese).(靳清贤, 李晶, 李孝刚, 张莉, 方少明, 刘鸣华, 化学进展, 2014, 26, 919.)(c) Yang, C. Chin. Chem. Lett. 2013, 24, 437.
[3] Tang, K. W.; Yi, J. M.; Huang, K. L.; Zhang, G. L. Chirality 2009, 21, 390.
[4] (a) Ma, F. N.; Ai, L.; Shen, X. M.; Zhang, C. Org. Lett. 2007, 9, 125.(b) Wang, W. G.; Shen, X. M.; Ma, F. N.; Li, Z. J.; Zhang, C. Tetrahedron: Asymmetry 2008, 10, 1193.(c) Wang, W. G.; Shen, X. M.; Zhang, C. Chin. J. Org. Chem. 2010, 30, 1126 (in Chinese).(王文革, 申秀民, 张聪, 有机化学, 2010, 30, 1126.)(d) Liu, F. L.; Li, Y. Y.; Wang, W. G.; Xu, J.; Liu, Y. P.; Xiao, Q. B. Chin. J. Org. Chem. 2011, 31, 747 (in Chinese).(刘丰良, 李媛媛, 王文革, 徐军, 刘言萍, 肖清波, 有机化学, 2011, 31, 747.)
[5] (a) Böhmer, V. Angew. Chem., Int. Ed. 1995, 34, 713.(b) Ikeda, A.; Shinkai, S. Chem. Rev. 1997, 97, 1713.(c) Harrowfield, J. Chem. Commun. 2013, 1578.(d) Casnati, A. Chem. Commun. 2013, 6827.(e) Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780.(f) Guo, D. S.; Liu, L. Chem. Soc. Rev. 2012, 41, 5907.(g) Yuan, Z.; Liang, F. Curr. Org. Chem. 2014, 18, 2016.(h) Li, L.; Qi, W. G.; Wang, C.; Yan, C. G. Chin. J. Org. Chem. 2013, 33, 1804 (in Chinese).(李亮, 戚伟光, 王超, 颜朝国, 有机化学, 2013, 33, 1804.)
[6] (a) Qing, G. Y.; Liu, S. Y.; He, Y. B. Prog. Chem. 2008, 20, 1933 (in Chinese).(卿光焱, 刘顺英, 何永炳, 化学进展, 2008, 20, 1933.)(b) Mutihac, L.; Lee, J. H.; Kim, J. S.; Vicens, J. Chem. Soc. Rev. 2011, 40, 2777.
[7] (a) Zheng, Y. S.; Zhang, C. Org. Lett. 2004, 6, 1189.(b) Zheng, Y. S.; Xiao, Q. Chin. J. Chem. 2005, 23, 1289.(c) Liu X. X.; Zheng, Y. S. Tetrahedron Lett. 2006, 47, 6357.
[8] Shirakawa, S.; Moriyama, A.; Shimizu, S. Org. Lett. 2007, 9, 3177.
[9] Liu, L.; Kou, Y. H.; Wang, L. Y.; Cao, D. R. Chin. J. Org. Chem. 2011, 31, 964 (in Chinese).(刘陆智, 寇玉辉, 汪凌云, 曹德榕, 有机化学, 2011, 31, 964.)
[10] (a) Li, Z. Y.; Chen, J. W.; Wang, L. Y.; Pan, Y. Synlett 2009, 2356.(b) Li, Z. Y.; Teng, M. Y.; Ma, J. J.; Huang, J.; Wang, L. Y.; Pan, Y. Sci. China, Ser. B: Chem. 2009, 52, 497.(c) Li, Z. Y.; Lu, C. X.; Huang, G. L.; Ma, J. J.; Sun, H. S.; Wang, L. Y.; Pan, Y. Lett. Org. Chem. 2010, 7, 461.(d) Li, Z. Y.; Xing, H. J.; Huang, G. L.; Sun, X. Q.; Jiang, J. L.; Wang, L. Y. Sci. Chin. Chem. 2011, 54, 1726.(e) Li, Z. Y.; Chen, J. W.; Liu, Y.; Xia, W.; Wang, L. Y. Curr. Org. Chem. 2011, 15, 39.
[11] Li, Z. Y.; Ma, J. J.; Chen, J. W.; Pan, Y.; Jiang, J. L.; Wang, L. Y. Chin. J. Chem. 2009, 27, 2031.
[12] (a) Huang, F. H.; Fronczek, F. R.; Gibson, H. W. J. Am. Chem. Soc. 2003, 125, 9272.(b) Cheng, P. N.; Lin, C. F.; Liu, Y. H.; Lai, C. C.; Peng, S. M.; Chiu, S. H. Org. Lett. 2006, 8, 435.
/
| 〈 |
|
〉 |