

Chinese Journal of Organic Chemistry >
Synthesis of Novel Hydroxyl-Functionalized Ionic Liquids andApplication in Knoevenagel Condensation
Received date: 2015-01-13
Revised date: 2015-03-19
Online published: 2015-03-24
Supported by
Project supported by the National Science and Technology Ministry (No. 2012BAK30B03) and the Fundamental Research Funds for the Central Universities (Nos. 2232013A3-05, CUSF-DH-D2013048).
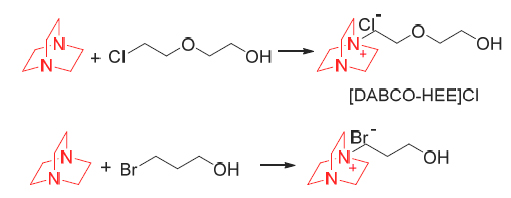
Knoevenagel condensation of benzaldehyde with active methylene compounds, such as ethylcyanoacetate and 2,4-thiazolidinedione proceeded very smoothly in novel hydroxyl-functionalized ionic liquids based on 1,4-diazabicyclo- [2.2.2]octane (DABCO) under solvent-free conditions and these ionic liquids afforded the products in excellent yields [β-benzyl-α-cyanoacrylicacidethylester (≥99%) and 5-benzylidene-2,4-thiazolidinedione (92%)]. These reactions were operated simply, and the desired products were separated directly from the reaction mixture without further purification. In addition, the ionic liquids used were regenerated and recycled several times without significant loss of activity. Finally, a plausible reaction mechanism was proposed, and the relevant evidence was given.
Key words: ionic liquids; hydroxyl-functionalized; Knoevenagel condensation
Liu Yuting , Li Rong , Xing Yanjun . Synthesis of Novel Hydroxyl-Functionalized Ionic Liquids andApplication in Knoevenagel Condensation[J]. Chinese Journal of Organic Chemistry, 2015 , 35(7) : 1520 -1525 . DOI: 10.6023/cjoc201501013
[1] Zhao, D.-W; Wu, Y.-T.; Chen, T.-T.; Dai, L.-Y.; Wang, Y.-Y. Chin. J. Org. Chem. 2013, 33, 1791 (in Chinese).(赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33, 1791.)
[2] Hou, H.-L.; Li, Z.-F.; Ying, A.-G. Chin. J. Org. Chem. 2014, 34, 1277 (in Chinese).(侯海亮, 李志峰, 应安国, 许松林, 有机化学, 2014, 34, 1277.)
[3] Peter, W.; Wilhelm, K. Angew. Chem., Int. Ed. 2000, 39, 3772.
[4] (a) Plechkova, N. V.; Seddon, K. R. Chem. Soc. Rev. 2008, 37, 123; (b) Tokuda, H.; Tsuzuki, S.; Susan, M. A. B. H.; Hayamizu, K.; Watanabe, M. J. Phys. Chem. B. 2006, 110, 19593.
[5] Fei, Z.-F.; Geldbach, T. J.; Zhao, D.-B.; Dyson, P. J. Chem. Eur. J. 2006, 12, 2122.
[6] Branco, L. C.; Rosa, J. N.; Ramos, J. J. M.; Afonso, C. A. M. A. Chem. Eur. J. 2002, 8, 3671.
[7] Dzyuba, S. V.; Bartsch, R. A. Tetrahedron Lett. 2002, 43, 4657.
[8] Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tertrahedron Lett. 2000, 41, 6993.
[9] Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967.
[10] Rumer, J. W.; Dai, S. Y.; Levick, M.; Biniek, L.; Procter, D. J.; McCulloch, I. J. Polym. Sci., Polym. Chem. 2013, 51, 1285.
[11] Han, J.-J.; Xu, Y.-F.; Su, Y.-P.; She, X.-G.; Pan, X.- F. Catal. Commun. 2008, 9, 2077.
[12] Shanthan, R. P.; Venkataratnam, R. V. Tetrahedron Lett. 1991, 32, 5821.
[13] Reddy, B. M.; Patil, M. K.; Rao, K. N.; Reddy, G. K. J. Mol. Catal. A: Chem. 2006, 258, 302.
[14] (a) Hu, Y.; Chen J.; Le Z. G.; Zheng, Q.-G. Synth. Commun. 2005, 35, 739.(b) Yue, C. B.; Mao, A. Q.; Wei, Y. Y.; Lü, M.-J. Catal. Commun. 2008, 9, 1571.
[15] Wu, K.; Li, C.-X. Chin. J. Org. Chem. 2011, 31, 119 (in Chinese).(吴坤, 李存雄, 有机化学, 2011, 31, 119.)
[16] Li, J.-Y.; Peng, J.-J.; Qiu, H.-Y.; Jiang, J.-X.; Wu, J.-R.; Ni, Y.; Lai, G.-Q. Chin. J. Org. Chem. 2007, 27, 483 (in Chinese).(厉嘉云, 彭家建, 邱化玉, 蒋剑雄, 邬继荣, 倪勇, 来国桥, 有机化学, 2007, 27, 483.)
[17] Li, J.; Sun, H.; Cai, X.-C.; Dai, L.-Y. Chin. J. Org. Chem. 2007, 27, 1296 (in Chinese).(李娟, 孙辉, 蔡晓晨, 戴立益, 有机化学, 2007, 27, 1296.)
[18] Morison, D.; Forbes, D. C.; Davis, J. H. Jr. Tetrahedron Lett. 2001, 42, 6053.
[19] Wang, Y.; Shang, Z. C.; Fan, T. X.; Chen, X. J. Mol. Catal. A 2006, 253, 212.
[20] Ying, A.-G.; Ni, Y.-X.; Xu, S.-L.; Liu, S.; Yang, J.-G.; Li, R.-R. Ind. Eng. Chem. Res. 2014, 53, 5678.
[21] AIST: Integrated Spectral Database System of Organic Compounds [Data were obtained from the National Institute of Advanced Industrial Science and Technology (Japan)].
[22] Xu, D.-Z.; Liu, Y.-J.; Shi, S.; Wang, Y.-M. Green Chem. 2010, 12, 514.
[23] Ying, A.-G.; Liu, L.; Wu, G.-F; Chen, X.-Z.; Ye, W.-D.; Chen, J.-H.; Zhang, K.-Y. Chem. Res. Chin. Univ. 2009, 25, 876.
[24] Xu, X.-M.; Li, Y.-Q.; Zhou, M.-Y. Chin. J. Org. Chem. 2004, 24, 1253 (in Chinese).(徐欣明, 李毅群, 周美云, 有机化学, 2004, 24, 1253.)
[25] Shelke, K. F.; Sapkal, S. B.; Madje, B. R.; Shingate, B. B.; Shingare, M. S. Bull. Catal. Soc. Ind. 2009, 8, 30.
[26] Jawale, D. V.; Pratap, U. R.; Lingampalle, D. L.; Mane, R. A. Chin. J. Chem. 2011, 29, 942
[27] Manabendra, S.; Sanchita, R.; Subrata Kumar, C.; Sanjay, B. Green Chem. Lett. Rev. 2008, 1, 113.
[28] Zhang, J.; Zhang, Y.-L.; Zhou, Z.-Q. Green Chem. Lett. Rev. 2014, 7, 90.
/
| 〈 |
|
〉 |