

Chinese Journal of Organic Chemistry >
Synthesis and Catalytic Esterification Performance of Caprolactam Task-Specific Ionic Liquids
Received date: 2015-03-10
Revised date: 2015-04-21
Online published: 2015-04-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20576026, 21106032).
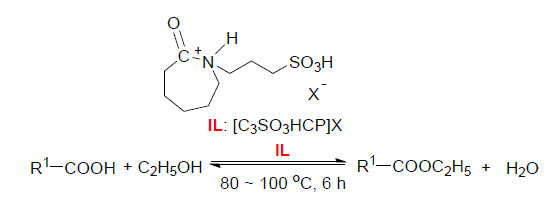
Four kinds of caprolactam task-specific ionic liquids (TSILs), 1-(3-sulfopropyl)caprolactam hydrogen sulfate, 1-(3-sulfopropyl)caprolactam p-toluene sulfonate, 1-(3-sulfopropyl)caprolactam phosphate sulfonate, 1-(3-sulfopropyl)ca-prolactam tetrafluoroborate, were prepared and characterized with low price caprolactam as raw material. The catalytic activities of these acidic ionic liquids were investigated by the esterification of acetic acid with ethanol and were compared with 3 kinds of SO3H-functionalized ionic liquids with different nitrogen heterocycles and concentrated sulfuric acid. The results showed that under the optimized conditions [n(C2H5OH):n(CH3COOH)=1:1.5], catalyst amount of 5% (w) based on acetic acid and ethanol., reaction temperature 80 ℃ and reaction time 6 h), the yield of ethyl acetate was up to 93.8%. The ionic liquids dried in vacuum till remained high activity after reused for 10 times, and the corrosion rates of the 316L austenitic stainless steel plates immersed in the systems with these ionic liquids were less than one sixth of that with sulfuric acid. Finally, the yields of series of ethyl esters were also high with [C3SO3HCP]HSO4 as catalyst, and the ionic liquids can form split-phases with ester product. Compared with the traditional sulfuric acid catalytic esterification, catalytic esterification of such ionic liquid has the advantages of low production cost, clean process, low corrosion rate, long useage cycle, and has the potential of replacing the traditional sulfuric acid in catalytic esterification reaction.
Hu Jingjing , Zhao Dishun , Li Jingjing , Zhai Jianhua , Hu Tiantian . Synthesis and Catalytic Esterification Performance of Caprolactam Task-Specific Ionic Liquids[J]. Chinese Journal of Organic Chemistry, 2015 , 35(8) : 1773 -1780 . DOI: 10.6023/cjoc201503012
[1] Li, X.; Lin, Q.; Ma, L. Ultrason. Sonochem. 2010, 17, 752.
[2] Zhao, D. S.; Liu, M. S.; Xu, Z. C.; F, J. T.; Ren, P. B. Chem. Ind. Eng. Prog. 2011, 30, 2287 (in Chinese). (赵地顺, 刘猛帅, 徐智策, 付江涛, 任培兵, 化工进展, 2011, 30, 2287.)
[3] Zhao, D. B.; Fei, Z. F.; Geldbach, T. J.; Scopelliti, R.; Laurenczy, G.; Dyson, p. J. J. Am. Chem. Soc. 2005, 88, 665.
[4] Inui, K.; Kurabayashi, T.; Sato, S.; Ichikawa, N. J. Mol. Catal. A: Chem. 2004, 216, 147.
[5] Zhao, D. S.; Liu, M. S.; Ge, J. J.; Zhang, J.; Ren, P. B. Chin. J. Org. Chem. 2012, 32, 2382 (in Chinese). (赵地顺, 刘猛帅, 葛京京, 张娟, 任培兵, 有机化学, 2012, 32, 2382.)
[6] Li, Y.; Hu, S. L.; Cheng, J. H.; Lou, W. Y. Chin. J. Catal. 2014, 35, 396.
[7] Song, Y. L.; Wang, X. C.; Huang, C. P.; Liang, F. B.; Liu, Z. C.; Chen, B. H. Chin. J. Org. Chem. 2013, 33, 1715 (in Chinese). (宋彦磊, 王新承, 黄崇品, 梁凤兵, 毓志超, 陈标华, 有机化学, 2013, 33, 1715.)
[8] Zhao, D. W.; Wu, Y. T.; Chen, T. T.; Dai, L. Y.; Wang, Y. Y. Chin. J. Org. Chem. 2013, 33, 1791 (in Chinese). (赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33, 1791.)
[9] Gui, J. Z.; Ban, H. Y.; Cong, X. H.; Zhang, X. T.; Hu, Z. D.; Sun, Z. L. J. Mol. Catal. A: Chem. 2005, 225, 27.
[10] Cai, X. J.; Cui, S. H.; Qu, L. P.; Yuan, D. D.; Lu, B.; Cai, Q. H. Catal. Commun. 2008, 9, 1173.
[11] Shi, F.; Zhang, Q. H.; Li, D. M.; Deng, Y. Q. Chem.-Eur. J. 2008, 11, 5279.
[12] Dawood, E.; Babak, K.; Abbas, M.; Javad, R. ChemPlusChem 2014, 79, 1147.
[13] James, H. C.; Thomas, J. F.; Duncan, J. M.; James, S. Sustainable Chem. Processes 2013, 1(13), 1.
[14] Han, X. X.; Du, H.; Hung, C. T.; Liu, L. L.; Wu, P. H.; Ren, D. H.; Huang, S. J.; Liu, S. B. Green Chem. 2015, 17(1), 499.
[15] Li, Z.; Zhao, Y. W.; Han, F.; Yang, L.; Song, H. Y.; Chen, J.; Xia, C. G. Sci. China: Chem. 2012, 42(4), 502 (in Chinese). (李臻, 赵应伟, 韩峰, 杨磊, 宋河远, 陈静, 夏春谷, 中国科学: 化学, 2012, 42(4), 502.)
[16] Swatloski, R. P.; Holbrey, J. D.; Memon, S. B.; Caldwell, G. A.; Caldwell, K. A. Rogers, R. D. Chem. Commun. 2004, 668.
[17] Du, Z.; Li, Z.; Zhang, J.; Zhu, L.; Deng, Y. J. Phys. Chem. B 2005, 109, 19542.
[18] Duran, A.; Jimenez De Haro, M. C.; Perezrodriguez, J. L.; Franqelo, M. L.; Herrera, L. K.; Justo, A. Appl. Surf. Sci. 2007, 253(17), 7295.
[19] Ilaria, P.; Ugo, B.; Stefano, C.; Alessio, F.; Alessandro, L. Sol. Energy Mater. Sol. Cells 2008, 92, 510.
[20] Lv, X. M. M. S. Thesis, Nanjing University, Nanjing, 2011 (in Chinese). (吕学铭, 硕士论文, 南京大学, 南京, 2011.)
[21] Tan, W. Y.; Zhang, Z. H.; Chen, Q.; He, M. Y. Chem. Ind. Eng. Prog. 2012, 31, 896 (in Chinese). (谭维一, 张致慧, 陈群, 何明阳, 化工进展, 2012, 31, 896.)
[22] Gao, H. B. Organic Chemistry, Higher Education Press, Beijing, 2005, p. 301 (in Chinese). (高鸿宾, 有机化学, 高等教育出版社, 北京, 2005, p. 301.)
[23] Ge, J. J. M. S. Thesis, Hebei University of Science and Technology, Hebei, 2014 (in Chinese). (葛京京, 硕士论文, 河北科技大学, 河北, 2014.)
[24] Huang, B. H.; Li, Z. J.; Shi, N.; Xu, X. L.; Fang, Y. X. Chin. J. Org. Chem. 2009, 29, 770 (in Chinese). (黄宝华, 黎子进, 史娜, 徐效陵, 张焜, 方岩雄, 有机化学, 2009, 29, 770.)
/
| 〈 |
|
〉 |