

Chinese Journal of Organic Chemistry >
Synthesis of Diarylaminofluorenes through Tandem Copper and Palladium Catalyzed Coupling-Cyclizing Reactions
Received date: 2015-03-26
Revised date: 2015-04-14
Online published: 2015-04-27
Supported by
Project supported by the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120074120008) and the Shanghai Key Laboratory of Molecular Catalysts and Innovative Materials (Fudan University, No. 2012MCIMKF01).
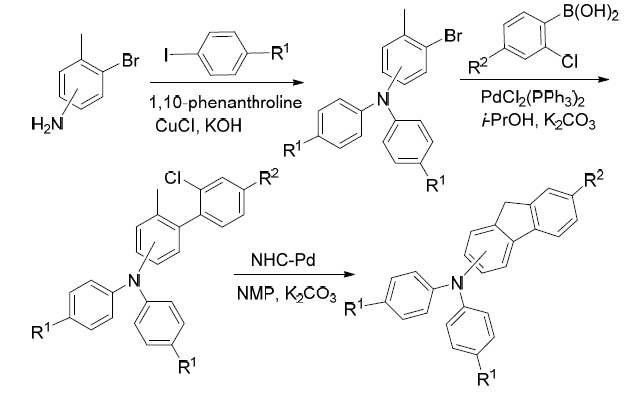
A convenient method of tandem copper and palladium catalyzed coupling-cyclizing reactions has been developed for the preparation of diarylaminofluorenes. Methylbromotriphenylamines prepared by the copper-catalyzed reaction of substituted iodobenzenes and methylbromoanilines, coupled with chlorophenylboronic acid under palladium catalyst to obtain the chlorophenylmethyltriphenylamines (V). A series of diarylaminofluorenes can be generated in good yields through palladium-catalyzed activation of benzylic C(sp3)—H bond of compound V. It was found that the 2-diarylaminofluorenes or 3-diarylaminofluorenes could be efficiently got. These compounds were characterized by 1H NMR, 13C NMR, HRMS, IR and UV-vis.
Key words: diarylaminofluorene; C—H activation; copper-catalyzed; palladium-catalyzed
Cai Liangzhen , Lin Hui , Liu Taoping , Tao Xiaochun . Synthesis of Diarylaminofluorenes through Tandem Copper and Palladium Catalyzed Coupling-Cyclizing Reactions[J]. Chinese Journal of Organic Chemistry, 2015 , 35(8) : 1682 -1690 . DOI: 10.6023/cjoc201503041
[1] Meng, H.; Zheng, J.; Lovinger, A. J.; Wang, B. C.; Van Patten, P. G.; Bao, Z. Chem. Mater. 2003, 15, 1778.
[2] Ahmed, S. A. M.; Al-Raqa, S. Y. J. Phys. Org. Chem. 2011, 24, 173.
[3] Higashijima, S.; Inoue, Y.; Miura, H.; Kubota, Y.; Funabiki, K.; Yoshida, T.; Matsui, M. RSC Adv. 2012, 2, 2721.
[4] Wu, Y.; Zhu, W. Chem. Soc. Rev. 2013, 42, 3453.
[5] Pei, J.; Liu, X. L.; Huang, W. Macromolecules 2003, 36, 323.
[6] Chen, X.; Tseng, H. E.; Liao, J.-L.; Chen, S.-A. J. Phys. Chem. B 2005, 109, 17496.
[7] Becker, K.; Lupton, J. M.; Feldmann, J.; Nehls, B. S.; Galbrecht, F.; Gao, D. Q.; Scherf, U. Adv. Funct. Mater. 2006, 16, 364.
[8] Lin, Y.; Chen, Y.; Gu, H. L.; Pan, Z.; Chen, J.-N. J. Funct. Polym. 2012, 25, 79 (in Chinese). (林楹, 陈彧, 顾慧丽, 潘喆, 陈军能, 功能高分子学报, 2012, 25, 79.)
[9] Jiao, S.-B.; Liao Y.; Xu, X.-J.; Wang, L.-P.; Yu, G.; Wang, L.-M.; Su, Z.-M.; Ye, S.-H.; Liu, Y.-Q. Adv. Funct. Mater. 2008, 18, 2335.
[10] Hao, Y.; Yang, X.-C.; Zhou, M.-Z.; Cong, J.-Y.; Wang, X.-N.; Hagfeldt, A.; Sun, L.-C. ChemSusChem 2011, 4, 1601.
[11] Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teuscher, J.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S. M.; Grätzel, M.; Bäuerle, P. Adv. Funct. Mater. 2012, 22, 1291.
[12] Cai, N.; Wang, Y.-L.; Xu, M.-F.; Fan, Y.; Li, R.-Z.; Zhang, M.; Wang, P. Adv. Funct. Mater. 2013, 23, 1846.
[13] Scrascia, A.; Marco, L. D.; Laricchia, S.; Picca, R. A.; Carlucci, C.; Fabiano, E.; Capodilupo, A. L.; Sala, F. D.; Gigli, G.; Ciccarella, G. J. Mater. Chem. A 2013, 1, 11909.
[14] Capodilupo, L. A.; Marco, L. D.; Fabiano, E.; Giannuzzi, R.; Scrascia, A.; Clarlucci, C.; Corrente, G. A.; Cipolla, M. P.; Gigli, G.; Ciccarella, G. J. Mater. Chem. A 2014, 2, 14181.
[15] Kobin, B.; Grubert, L.; Blumstengel, S.; Henneberger, F.; Hecht, S. J. Mater. Chem. 2012, 22, 4383.
[16] Song, Y.; Xu, W.; Zhu, D. Tetrahedron Lett. 2010, 51, 4894.
[17] Liu, T.-P.; Sun, J.; Li, R.; Tao, X.-C. Chin. J. Org. Chem. 2011, 31, 1799 (in Chinese). (刘涛平, 孙杰, 李瑞, 陶晓春, 有机化学, 2011, 31, 1799.)
[18] Kelkar, A.; Patil, M.; Chaudhari, R. V. Tetrahedron Lett. 2002, 43, 7143.
[19] Tsuji, J. Palladium Reagents and Catalysts, John Wiley & Sons Ltd., Chichester, 2004, p. 289.
[20] Tao, X.-C.; Zhang, Y.-P.; He, T.-X.; Shen, D. Chin. J. Chem. 2007, 25, 1326.
[21] Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem. 2009, 121, 5196.
[22] Kakiuchi, F.; Chatani, N, Adv. Synth. Catal. 2003, 345, 1077.
[23] Hu, Q.-S. Synlett 2007, 1331.
[24] Liu, T.-P.; Xing, C.-H.; Hu, Q.-S. Angew. Chem., Int. Ed. 2010, 49, 2909.
[25] Herrmann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1290.
[26] Selvakumar, A.; Zapf, A.; Beller, M. Org. Lett. 2002, 4, 3031.
[27] Yang, C.; Lee, H. M.; Nolan, S. P. Org. Lett. 2001, 3, 1511.
[28] Hsiao, C.-C.; Lin, Y.-K.; Liu, C.-J.; Wu, T.-C.; Wu, Y.-T. Adv. Synth. Catal. 2010, 352, 3267.
[29] Chaumontet, M.; Piccardi, R.; Audic, N.; Hitce, J.; Peglion, J.-L.; Clot, E.; Baudoin, O. J. Am. Chem. Soc. 2008, 130, 15157.
[30] Qiu, Y.; Li, Y.-K.; Dong, H.; Duan, L.; Gao, Y.-D. CN 1931803, 2006 [Chem. Abstr. 2007, 146, 430917].
[31] Kang, J.-S.; Park, J. H.; Jun, S. W.; Shin, Y.-J.; Chang, Y.-M.; Yang, N.-C.; Park, J.-K.; Lee, S. WO 2014021572, 2014 [Chem. Abstr. 2014, 160, 319895].
[32] Li, X.-L.; Wu, W.; Fan, X.-H.; Yang, L.-M. RSC Adv., 2013, 3, 12091.
[33] Kang, J.-S.; Park, J. H.; Jun, S. W.; Shin, Y.-J.; Chang, Y.-M.; Yang, N.-C.; Park, J.-K.; Lee, S. WO 2014073791, 2014 [Chem. Abstr. 2014, 160, 714102].
[34] Wang, M.; Nalla, V.; Jeon, S.; Mamidala, V.; Ji, W.; Tan, L.-S.; Cooper, T.; Chiang, L.-Y. J. Phys. Chem. C Nanomater Interfaces 2011, 115, 18552.
[35] Ishida, T.; Mochizuki, F.; Yabuki, S.; Sawashita, Y.; Kurachi, M. JP 2005338446, 2005 [Chem. Abstr. 2005, 144, 14117].
[36] Senoo, A.; Yashiro, R.; Kikuchi, N.; Kanamaru, T. JP 03061952, 1989 [Chem. Abstr. 1991, 115, 170924].
/
| 〈 |
|
〉 |