

Chinese Journal of Organic Chemistry >
Synthesis and Properties of Novel Tripod Type Multi-benzimidazole Quaternary Ammonium Compounds
Received date: 2014-12-08
Revised date: 2015-03-25
Online published: 2015-04-30
Supported by
Project supported by the Science and Technology Research Program of Liaoning Provincial Department of Education (No. 2009A426).
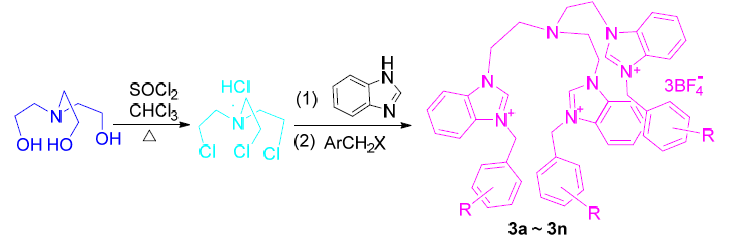
Fourteen novel tripod type multi-benzimidazole quaternary ammoniums (3a~3n) with complex functions were synthesized for the first time and the structures of the target molecules were well characterized. The inhibitory activities of Cdc25B and PTP1B of 3 were explored. The results showed that 3d, 3e, 3i, 3j and 3l exhibit excellent inhibitory activity against Cdc25B, wherein the IC50 values of 3d, 3e, 3j and 3l were lower than the reference Na3VO4, which were expected to be potential Cdc25B inhibitors for the treatment of cancer. Compounds 3d, 3e, 3i and 3l showed good inhibitory activity against PTP1B, wherein the IC50 value of 3l was lower than the reference oleanolic acid, which may be used as a potential PTP1B inhibitor for the treatment of diabetes. Meanwhile, the fluorescence quenching of six kinds of anions on four target compounds was also studied. It was found that I-exhibited excellent quenching effect, 3c and 3e behaved the best quenching effect and may be a potential recognition fluorescent I-sensor probe.
Key words: benzimidazole; drug activity; fluorescent sensor probe; CDc25B; PTP1B
Zhang Chenglu , Guo Yang , Chen Ying , Sun Lijie , Cheng Anqi , Zhao Na , Zhao Baocheng , Tang Jie , Xi Huan . Synthesis and Properties of Novel Tripod Type Multi-benzimidazole Quaternary Ammonium Compounds[J]. Chinese Journal of Organic Chemistry, 2015 , 35(8) : 1665 -1672 . DOI: 10.6023/cjoc201412014
[1] Song, L.; Xie, Y. Y.; Wang, H. Z. Chin. J. Med. Chem. 2000, 92. (in Chinese). (宋琳, 谢毓元, 王惠贞, 中国药物化学杂志, 2000, 92.)
[2] Meng, J. P.; Lu, Y. H.; Halqam, I.; Zhou, C. H. Chin. J. Biochem. Pharmacol. 2008, 29, 418. (in Chinese). (孟江平, 卢一卉, 海力且木·依不那音, 周成合, 中国生化药物杂志, 2008, 29, 418.)
[3] Guo. Y. C.; Zhou, L. H.; Qiao. Z. P. Chin. J. Inorg. Chem. 2006, 134 (in Chinese). (郭应臣, 卓立宏, 乔占平, 无机化学学报, 2006, 134.)
[4] Ören, I.; Temiz, Ö.; Yalc?n, I.; ?ener, E.; Altanlar, N. Eur. Pharm. Sci. 1989, 7, 153.
[5] Klimešová, V.; Ko??á, J.; Pour, M.; Stachel, J.; Waisser, K.; Kaustová, J. Eur. Med. Chem. 2002, 33, 137.
[6] Ansari, K. F.; Lal, C. Eur. J. Med. Chem. 2009, 44, 2294.
[7] Rajakumara, P.; Sekara, K.; Shanmugaiahb, V.;, Mathivananb, N. Bioorg. Med. Chem. Lett. 2008, 18, 4416.
[8] Beaulieu, P. L.; Bousquet, Y.; Gauthier, J.; Gillard, J.; Marquis, M.; McKercher, G.; Pellerin, C.; Valois, S.; Kukolj, G. J. Med. Chem. 2004, 47, 6884.
[9] Hirashima, S.; Suzuki, T.; Ishida, T.; Noji, S.; Yata, S.; Ando, I.; Komatsu, M.; Ikeda, S.; Hashimoto, H. T. J. Med. Chem. 2006, 49, 4721.
[10] Asthana, S.; Shukla, S.; Vargiu, A. V. Biochemistry 2013, 52, 3752.
[11] Li, Y. F.; Wang, G. F.; He, P. L.; Huang, W. G.; Zhu, F. H. ; Gao, H. Y.; Tang, W.; Luo, Y.; Feng, C. L.; Shi, Li. P.; Ren, Y. D.; Lu, W.; Zuo, J. P. J. Med. Chem. 2006, 49, 4790.
[12] Fang, X. P.; Mei, X. G. Foreign Med. Sci. 2005, 32, 115 (in Chinese). (方学平, 梅兴国, 国外医学, 2005, 32, 15.)
[13] Combs, A. P.; Zhu, W. Y.; Crawley, M. L.; Glass, B.; Polam, P.; Sparks, R. B.; Modi, D.; Takvorian, A.; McLaughlin, E.; Yue, E. W.; Wasserman, Z.; Bower, M.; Wei, M.; Rupar, M.; Ala, P. J.; Reid, B. M.; Ellis, D.; Gonneville, L.; Emm, T.; Taylor, N.; Yeleswaram, S.; Li Y. L.; Richard, W.; Timothy, C. B.; Gregory, H.; Phillip, C. C.; Liu, B. M. J. Med. Chem. 2006, 49, 3774.
[14] Ong, J. X.; Yap, C. W.; Ang, W. H. Inorg. Chem. 2012, 51, 12483.
[15] Erion, M. D.; Dang, Q.; Reddy, M. R.; Kasibhatla, S. R.; Huang, J. W.; Lipscomb, W. N.; Poelje, P. D. J. Am. Chem. Soc. 2008, 129, 15480.
[16] Dang, Q.; Kasibhatla, S. R.; Xiao, W.; Liu, Y.; DaRe, J.; Taplin, F.; Reddy, K. R.; Scarlato, G. R.; Gibson, T.; Poelje, P. D.; Potter, S. C.; Erion, M. D. J. Med. Chem. 2010, 53, 441.
[17] Settimo, F. D.; Primofiore, G.; Settimo, A. D. J. Med. Chem. 2001, 44, 4359.
[18] Ramla, M. M.; Omar, M. A.; El-Khamry, A-M. M.; El-Diwani, H. I. Bioorg. Med. Chem. 2006, 14, 7324.
[19] Rewcastle, G. W.; Gamage, S. A.; Flanagan, J. U. J. Med. Chem. 2011, 54, 105.
[20] Lavecchia, A.; Giovanni, C. D.; Pesapane, A.; Montuori, N.; Ragno, P.; Martucci, N. M.; Masullo, M.; Vendittis, E. D.; Novellino, E. J. Med. Chem. 2012, 55, 4142.
[21] Zhang, C. L.; Wang, X.; Guo, Y.; Wu, Y. F.; Gao, L. N.; Sun, L. J. Chai, J. H.; Zhu, C. A. Chin. J. Org. Chem. 2014, 34, 2331 (in Chinese). (张成路, 王雪, 国阳, 吴一非, 高丽娜, 孙丽杰, 柴金华, 朱长安, 有机化学, 2014, 34, 2331.)
[22] Vladislav, Y. K.; Igor, B. K. ; Vyacheslav, Y. S. Heteroat. Chem. 2005, 16, 492.
[23] Zhang, C. L.; Sun, L. J.; Wu, F. Y.; Qu, R. F.; Zhu, C. A.; Wang, X.; Chai, J. H.; Guo, Y.; Hu, X. Chem. J. Chin. Univ. 2014, 35, 1445 (in Chinese). (张成路, 孙立杰, 武飞宇, 曲瑞峰, 朱长安, 王雪, 柴金华, 国阳, 胡雪, 高等学校化学学报, 2014, 35, 1445.)
[24] Fukuyama, Y.; Tanimura, R.; Maeda, K. Anal. Chem. 2012, 84, 4237.
[25] Singh, N.; Jang, D. O. Org. Lett. 2007, 9, 1991.
[26] Martinez, M.; Scancenon, F. Chem. Rev 2003, 103, 4419.
[27] Kim, Y. H.; Sun, G. Text. Res. J. 2001, 71, 318.
[28] Kar, C.; Basu, A.; Das, G. Tetrahedron 2012, 53, 4754.
[29] Zhang, Y. N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J. Y.; Li, J.; Hua, L. H. Bioorg. Med. Chem. 2008, 16, 8697.
[30] Huang, W. G.; Jiang, Y. Y.; Li, Q.; Li, J.; Li, J. Y.; Lu, W.; Cai, J. C. Tetrahedron 2005, 61, 1863.
[31] Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
[32] Alam, M. M.; Nazreen, S.; Haider, S.; Shafi, S.; Yar, M. S.; Hamid, H.; Alam, M. S. Pharm. Chem. Life Sci. 2011, 45, 11
[33] Khatri, V. K.; Chahar, M.; Pavani, K.; Pandey, P. S. J. Org. Chem. 2007, 72, 10224.
[34] Boye, B.; Keramanea, E. M.; Monterob, J. L.; Roquea, J. P. Synth. Commun. 1998, 28, 1737.
[35] Wang, Z. T.; Zhu, L.; Yin, F.; Su, Z. Q.; Li, Z. D.; Li, C. Z. J. Am. Chem. Soc. 2012, 134, 4258.
[36] Matsuo, J.; Iida, D.; Yamanakaa, H.; Mukaiyamaa, T. Tetrahedron 2003, 59, 6739.
[37] Sun, J.; Feng, X. J.; Zhao, Z. R.; Yamamoto, Y.; Bao, M. Tetrahedron 2014, 70, 7166.
[38] Niknam, K.; Kiasat, A. R.; Kazemi, F.; Hossienia, A. Phosphorus, Sulfur, Silicon Relat. Elem.2003, 178, 1385.
[39] Li, Z.; Sheng, C.; Qiu, H.; Zhang, Y. The New J. Org. Synth. 2009, 39, 412.
[40] Zhang, J.; Cui, H. L.; Hojo, M.; Shuang, S. M.; Dong, C. Bioorg. Med. Chem. Lett. 2012, 22, 343.
[41] Caliendoa, G.; Fiorinoa, F.; Perissuttia, E.; Severinoa, B.; Gessib, S.; Cattabrigab, E.; Boreab, P. A.; Santagadaa, V. Eur. J. Med. Chem. 2001, 36, 873.
[42] Boger, D. L.; Palanki, M. S. S. J. Am. Chem. Soc. 1992, 114, 9318.
[43] Tan, H. H.; Ong, W. M.; Lai, S. H. Singapore Med. J. 2007, 48, 582.
/
| 〈 |
|
〉 |