

Chinese Journal of Organic Chemistry >
An Efficient Synthesis of Cytosporone B
Received date: 2015-03-30
Revised date: 2015-05-04
Online published: 2015-05-06
Supported by
Project supported by the National Basic Research Program (973 Program, No. 2010CB833802), the National Natural Science Foundation of China (Nos. 81373304, 91313303, 81302666), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13028) and the National Science Found for Distinguished Young Scholars of China (No. 30325044).
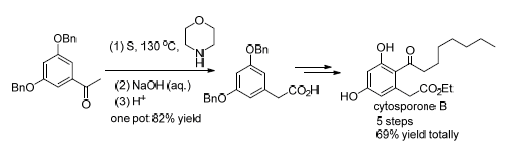
The fungal polyketide cytosporone B (Csn-B) displays diverse biological activities, and is an important small molecule probe for the studies on biological progress thus that its efficient synthesis attracts much attention. In this study, the commercial available 1-(3,5-dihydroxyphenyl)ethanone was used as starting material. After converting to benzyl ether, the product 1-(3,5-dibenzyloxyphenyl)ethanone was treated with sulfur powder and morpholine followed by hydrolysis in one pot to afford the key intermediate 3,5-dibenzyloxyphenylacetic acid via Willgerodt-Kindler reaction. Respectively, the 3,5-dibenzyloxyphenylacetic acid was converted to Csn-B or secocurvularin (67.3% overall yield) through esterification, Friedel-Crafts acylation reaction and deprotection procedures in 5 steps totally. Cytosporone A (Csn-A) was obtained in 65.3% yield through hydrolysis of Csn-B and cytosporone C (Csn-C) was provided in 64.0% yield via reduction-cyclization of Csn-B. Our synthetic approach to Csn-B and its derivatives has the advantages of short pathways, high yield, economical starting material, and is suitable for large-scale preparation.
Lü Chao, Li Jianfang, Xu Hongjiao, Shen Yuemao . An Efficient Synthesis of Cytosporone B[J]. Chinese Journal of Organic Chemistry, 2015 , 35(9) : 2013 -2018 . DOI: 10.6023/cjoc201503049
[1] Brady, F. S.; Wagenaar, M. M.; Singh, M. P.; Janso, J. E.; Clardy, J. Org. Lett. 2000, 2, 4043.
[2] Zhan, Y. Y.; Du, X. P.; Chen, H. Z.; Liu, J. J.; Zhao, B. X.; Huang, D. H.; Li, G. D.; Xu, Q. Y.; Zhang, M. Q.; Weimer, B. C.; Chen, D.; Cheng, Z.; Zhang, L. R.; Li, Q. X.; Li, S. W.; Zheng, Z. H.; Song, S. Y.; Huang, Y. J.; Ye, Z. Y.; Su, W. J.; Lin, S. C.; Shen, Y. M.; Wu, Q. Nat. Chem. Biol. 2008, 4, 548.
[3] Xia, Z. B.; Cao, X. H.; Rico-Bautista, E.; Yu, J. H.; Chen, L. Q.; Chen, J. B.; Bobkov, A.; Wolf, D. A.; Zhang, X. K.; Dawson, M. I. Med. Chem. Commun. 2013, 4, 332.
[4] Li, J. F.; Lv, C.; Sun, W. Y.; Li, Z. Y.; Han, X. W.; Li, Y. Y.; Shen, Y. M. Antimicrob. Agents Chemother. 2013, 57, 2191.
[5] Palumbo-Zerr, K.; Zerr, P.; Distler, A.; Fliehr, J.; Mancuso, R.; Huang, J.; Mielenz, D.; Tomcik, M.; Fürnrohr, B. G.; Scholtysek, C.; Dees C.; Beyer, C.; Krönke, G.; Metzger, D.; Distler, O.; Schett, G.; Distler, J. H. W. Nat. Med. 2015, 21, 150.
[6] Liu, J. J.; Zeng, H. N.; Zhang, L. R.; Zhan, Y. Y.; Chen, Y.; Wang, Y.; Wang, J.; Xiang, S. H.; Liu, W. J.; Wang, W. J.; Chen, H. Z.; Shen, Y. M.; Su, W. J.; Huang, P. Q.; Zhang, H. K.; Wu, Q. Cancer Res. 2010, 70, 3628.
[7] Wang, W. J.; Wang, Y.; Chen, H. Z.; Xing, Y. Z.; Li, F. W.; Zhang, Q.; Zhou, B.; Zhang, H. K.; Zhang, J.; Bian, X. L.; Li, L.; Liu, Y.; Zhao, B. X.; Chen, Y.; Wu, R.; Li, A. Z.; Yao, L. M.; Chen, P.; Zhang, Y.; Tian, X. Y.; Beermann, F.; Wu, M.; Han, J. H.; Huang, P. Q.; Lin, T. W.; Wu. Q. Nat. Chem. Biol. 2014, 10, 133.
[8] Zhan, Y. Y.; Chen, Y. Y.; Zhang, Q.; Zhuang, J. J.; Tian, M.; Chen, H. Z.; Zhang, L. R.; Zhang, H. K.; He, J. P.; Wang, W. J.; Wu, R.; Wang, Y.; Shi, C. F.; Yang, K.; Li, A. Z.; Xin, Y. Z; Li, T. Y.; Yang, J. Y.; Zheng, Z. H.; Yu, C. D.; Lin, S. C.; Chang, C. S.; Huang, P. Q.; Lin, T. W.; Wu, Q. Nat. Chem. Biol. 2012, 8, 897.
[9] Abrell, L. M.; Borgeson, B.; Crews, P. Tetrhedron Lett. 1996, 37, 8983.
[10] Huang, H.; Zhang, L.; Zhang, X. D.; Ji, X.; Ding, X.; Shen, X.; Jiang, H. L.; Liu, H. Chin. J. Chem. 2010, 28, 1041.
[11] Yoshida, H.; Morishita, T.; Ohshita, J. Chem. Lett. 2010, 39, 508.
[12] Delius, M.; Le, C. M.; Dong, V. M. J. Am. Chem. Soc. 2012, 134, 15022.
[13] Zamberlam, C. E. M.; Meza, A.; Leite, C. B.; Marques, M. R.; Limaa, D. P.; Beatriz, A. J. Braz. Chem. Soc. 2012, 23, 124.
[14] Priebbenow, D. L.; Bolm, Carsten. Chem. Soc. Rev. 2013, 42, 7870.
[15] Vujjini, S. K.; Krishnam, V. R. R. D.; Badarla, K. R.; Vetukuri, V. N. K. V. P. R.; Bandichhor, R.; Kagga, M.; Cherukupally, P. Tetrahedron Lett. 2014, 55, 3885.
[16] Li, X.; Reuman, M.; Russell, R. K.; Adams, R.; Ma, R.; Beish, S.; Branum, S.; Youells, S.; Roberts, J.; Jain, N.; Kanojia, R.; Sui, Z. Org. Process. Res. Dev. 2007, 11, 414.
[17] Bracher, F.; Krauss, J. Nat. Prod. Lett. 1998, 12, 31.
[18] Cai, Z. Y.; Hector, F. D. L. Chin. J. Org. Chem. 2014, 34, 1582 (in Chinese).(蔡祖恽, Hector F. DeLuca, 有机化学, 2014, 34, 1582.)
[19] Alam, M. M.; Adapa, S. R. Synth. Commun. 2003, 33, 59.
[20] Wu, W.; Huang, J. J. Org. Chem. 2014, 79, 10189.
[21] Chen, W.; Hao, H. L.; Zhang, C. L.; Shen, Y. M. Chin. J. Org. Chem. 2014, 34, 797 (in Chinese).(陈旺, 郝慧琳, 张晨露, 沈月毛, 有机化学, 2014, 34, 797.)
/
| 〈 |
|
〉 |