

Chinese Journal of Organic Chemistry >
Synthesis and Properties of Extended Bis-1,2-dithiolene Nickel Complexes Substituted with Unsaturated Group
Received date: 2015-03-04
Revised date: 2015-05-05
Online published: 2015-05-14
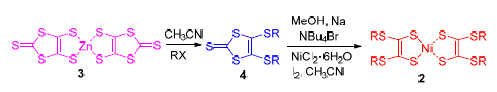
Three bis-1,2-dithiolene nickel complexes with unsaturated substituted groups in the terminals, bis[4,5-di(4'-t-butylbenzyl)thio-1,3-dithion]nickel (2a), bis[4,5-dially-thio-1,3-dithion]nickel (2b) and bis[4,5-di(3'-phenyl-ally)thio-1,3-di-thion]nickel (2c) together with a bis-1,2-dithiolene nickel complexes with saturated substituted groups in the terminals, bis[4,5-didecylthio-1,3-dithion]nickel (2d), as a reference, were synthesized and characterized by 1H NMR and MS. They were easily soluble in toluene with the maximum absorption wavelengths (λmax) around 1000 nm, the effects of substituent groups in the terminals on λmax were not so significant. Their λmax in solid polymethyl methacrylate (PMMA) were around 1000 nm as well, and no broadening absorption bands could be observed in lower concentration. The solid cyclic voltammetry (CV) curves showed that the oxidation potentials would be increased by introduction of phenyl group in the terminals, thus their electrochemical stability could be improved. The results of thermal gravimetric analysis (TGA) indicated that the temperature of 5% weight-loss was higher than 200 ℃ for these compounds. Irradiated by xenon lamp (300 W) for 100 min, their absorbance was decreased less than 100% in toluene and 2% in solid polymethyl methacrylate (PMMA), respectively. The introduction of phenyl group in the terminals could improve the photo-stability for these molecules both in solution and solid PMMA.
Sun Jian , Jin Zheming , Wang Chengyun , Shen Yongjia . Synthesis and Properties of Extended Bis-1,2-dithiolene Nickel Complexes Substituted with Unsaturated Group[J]. Chinese Journal of Organic Chemistry, 2015 , 35(9) : 1948 -1954 . DOI: 10.6023/cjoc201503005
[1] Men, J. F.; Cheng, H. F.; Chen, Z. H.; Chu, Z. Y. Mater. Rev. 2008, 22, 3 (in Chinese). (门金凤, 程海峰, 陈朝辉, 楚增勇, 材料导报, 2008, 22, 3.)
[2] Marshall, K. L.; Painte, G.; Lotito, K. A. Mol. Cryst. Liquid Cryst. 2006, 47, 449.
[3] Aragoni, C.; Arca, M.; Demartin, F.; Devillanova, F. A.; Garau, A.; Isaia, F.; Lelj, F.; Lippolis, V.; Verani, G. J. Am. Chem. Soc. 1991, 121, 7098.
[4] Mueller-Westerhoff, U. T.; Vance, B.; Yoon, D. I. Tetrahedron 1991, 47, 909.
[5] Espine, P. Coord. Chem. Rev. 1992, 117, 215.
[6] Fabian, J.; Nakazumi, H.; Matsuoka, M. Chem. Rev. 1992, 92(6), 1200.
[7] Coe, B. J. Compr. Coord. Chem. 2004, 9, 621.
[8] Cho, J. Y.; Domercq, B.; Jones, S. C.; Yu, J.; Zhang, X.; An, Z.; Bishop, M.; Barlow, S.; Marder, S. R.; Kippelen, B. J. Mater. Chem. 2007, 17, 2642.
[9] Stiefel, E. I. Karlin, K. D. Dithiolene Chemistry, Progress in Inorganic Chemistry, John Wiley Sons, 2004, 52, 399.
[10] Deplano, P.; Pilia, L.; Espa, D.; Mercuri, M. L.; Serpe, A. Coord. Chem. Rev. 2010, 254, 1434.
[11] Robertson, N.; Cronin, L. Coord. Chem. Rev. 2002, 227, 93.
[12] Chen, H.; Cheng, Z. S. J. East China Institute Chem. Technol. 1992, 18(5), 628 (in Chinese). (陈红, 程铸生, 华东化工学院学报, 1992, 18(5), 628.)
[13] Men, J. F.; Cheng, H. F.; Chen, Z. H.; Chu, Z. Y.; Zheng, W. W.; Wang, Q. Infrared Technol. 2008, 30, 150 (in Chinese).(门金凤, 程海峰, 陈朝辉, 楚增勇, 郑文伟, 王茜, 红外技术, 2008, 30, 150.)
[14] Dai, F.; Fan, W. H.; Wu, J. H.; Wang, L. X.; Zhang, Q. T. Laser Infrared 2011, 41, 1131 (in Chinese). (戴峰, 樊卫华, 吴金华, 王丽熙, 张其土, 激光与红外, 2011, 41(10), 1131.)
[15] Gao, J. X.; Miao, Q. Q.; Wang, Z. Q.; Gao, Y. R.; Ma, Y. L. Fine Chem. 2011, 28, 909 (in Chinese). (高俊雄, 苗青青, 王泽清, 高玉荣, 马廷丽, 精细化工, 2011, 28, 909.)
[16] Charlton, A.; Hill, C. A. S.; Underhill, A. E.; Malik, K. M. A.; Hursthouse, M. B.; Karaulovb, A. I.; Meller, J. J. Mater. Chem. 1994, 4(12), 1861.
[17] Robertson, N.; Cronin, L. Coord. Chem. Rev. 2002, 227, 93.
[18] Svenstrup, N.; Becher, J. Synthesis 1995, 215.
[19] Bui, T. T.; Vuong, M. H. New J. Chem. 2012, 36, 2033.
[20] Huang, Q.; Wang, L. X.; Zhang, Q. S. Laser Infrared 2008, 38(5), 421 (in Chinese). (黄强, 王丽熙, 张其土, 激光与红外, 2008, 38(5), 421.)
/
| 〈 |
|
〉 |