

Chinese Journal of Organic Chemistry >
Research Progress on the Synthesis of Brazilin-Type Natural Products
Received date: 2015-03-17
Revised date: 2015-05-03
Online published: 2015-05-14
Supported by
Project supported by the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13095), the National Natural Science Foundation of China (Nos. 21462049, 21332007, U1402227), the Natural Science Foundation of Yunnan Province (Nos. 2013FA028, 2012FB113) and the Excellent Young Talents of Yunnan University.
Brazilin-type natural products were isolated from the heartwood of Caesalpinia sappan, a famous traditional Chinese medicine. These compounds have stimulated a great deal of interest on synthetic chemists due to their structural novelty and promising biological activities. Up to now, many methods towards the synthesis of these natural products have been developed. Progress on the synthesis of Brazilin-type natural products are reviewed in this paper.
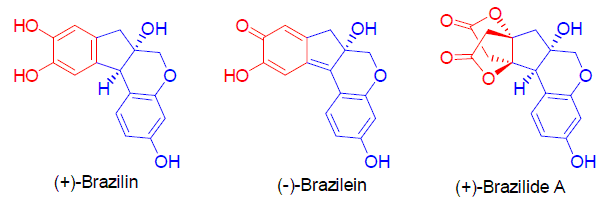
Key words: brazilin; brazilide A; brazilein; synthesis
Wang Xuequan , Liu Wei , Duan Suyue , Yang Xiaodong , Zhang Hongbin . Research Progress on the Synthesis of Brazilin-Type Natural Products[J]. Chinese Journal of Organic Chemistry, 2015 , 35(8) : 1585 -1597 . DOI: 10.6023/cjoc201503025
[1] Robinson, R. Bull. Soc. Chim. Fr. 1958, 125.
[2] Masahiro, N.; Seiji, N. Chem. Pharm. Bull. 1990, 38, 1490.
[3] Michio, N.; Hiroyuki, N.; Mariko, N. Chem. Pharm. Bull. 1987, 35, 3568.
[4] Yang, B. O.; Ke, C. Q.; He, Z. S.; Yang, Y. P.; Ye, Y. Tetrahedron Lett. 2002, 43, 1731.
[5] Kim, D. S.; Baek, N. I.; Oh, S. R.; Jung, K. Y.; Lee, I. S.; Lee, H. K. Phytochemistry 1997, 46, 177.
[6] (a) Puchtler, H.; Sweat, F. Histochemistry 1964, 4, 197. (b) Puchtler, H.; Meloan, S. N.; Waldrop, F. S. Histochemistry 1986, 85, 353.
[7] Kim, B. H.; Kim, S. J.; Jeong, E. J.; Sohn, J. H.; Jung, M.; Lee, H.; Kim, S. H. J. Agric. Food Chem. 2012, 60, 9882.
[8] (a)Kim, S. G.; Kim, Y. M.; Khil, L. Y.; Jeon, S. D.; So, D. S.; Moon, C. H.; Moon, C. K. Arch. Pharm. Res. 1998, 21, 140. (b) Hwang, G. S.; Kim, J. Y.; Chang, T. S.; Jeon, S. D.; So, D. S.; Moon, C. K. Arch. Pharm. Res. 1998, 21, 774.
[9] Hikino, H.; Taguchi, T.; Fujimura, H.; Hiramatsu, Y. Planta Med. 1977, 31, 214.
[10] Xu, H. X.; Lee, S. F. Phytother. Res. 2004, 18, 647.
[11] Moon, C. K.; Park, K. S.; Kim, S. G.; Won, H. S.; Chung, J. H. Drug Chem. Taxicol. 1992, 15, 81.
[12] Lee, Y. R.; Noh, E. M.; Han, J. H.; Kim, J. M.; Hwang, J. K.; Hwang, B. M.; Chung, E. Y.; Kim, B. S.; Lee, S. H.; Lee, S. J.; Kim, J. S. Eur. J. Pharmacol. 2012, 674, 80.
[13] Wang, X.; Zhao, H. X.; Bai, H. Acta Chin. Med. Pharmacol. 2013, 41, 164 (in Chinese). (王鑫, 赵焕新, 白虹, 中医药学报, 2013, 41, 164.)
[14] (a) Zhao, Y. N.; Pan, Y.; Tao, J. L.; Xing, D. M.; Du, L. J. Pharmacology 2006, 76, 76. (b) Zhong, X.; Wu, B.; Pan, Y. J.; Zheng, S. Neoplasma 2009, 56, 387. (c) Shen, J.; Zhang, H.; Lin, H.; Su, H.; Xing, D.; Du, L. Eur. J. Pharmacol. 2007, 558, 88. (d) Ye, M.; Xie, W. D.; Lei, F.; Meng, Z.; Zhao, Y. N.; Su, H.; Du, L. J. Int. Immunopharmacol. 2006, 6, 426. (e) Shen, J.; Yip, S.; Wang, Z. X.; Wang, W.; Xing, D. M.; Du, L. J. Eur. J. Pharmacol. 2008, 580, 366.
[15] Hill, R. A.; Parker, M. C. Nat. Prod. Rep. 2002, 19, ⅲ.
[16] Heller, W.; Tamm, C. Progress in the Chemistry of Organic Natural Products, Vol. 40, Eds.: Herz, H.; Criesenbach, H.; Kirby, W, Springer-Verlag, Inc., New Work, 1980, p.105.
[17] Saitoh, T.; Sakashita, S.; Nakata, H.; Shimokawa, T.; Kinjo, J.; Yamahara, J.; Yamasakim, M.; Nohara, T. Chem. Pharm. Bull. 1986, 34, 2506.
[18] (a) Kirkiacharian, B. S. C. R. Acad. Ser. C 1972, 274, 2096. (b) Kirkiacharian, B. S.; Billet, D.; Durgeat, M.; Heitz, S.; Adjangba, M. K. Bull. Soc. Chim. Fr. 1975, 1770.
[19] Yen, C. T.; Kyoko, N. G.; Hwang, T. L.; Wu, P. C.; Morris-Natschke, S. L.; Lai, W. C.; Bastowd, K. F.; Chang, F. R.; Wu, Y. C.; Lee, K. H. Bioorg. Med. Chem. Lett. 2010, 20, 1037.
[20] (a) Davis, F. A.; Chen, B. C. J. Org. Chem. 1993, 58, 1751. (b) Davis, F. A.; Chen, B. C. Tetrahedron Lett. 1990, 31, 6823.
[21] Arnoldi, A.; Bassoli, A.; Borgonovo, G.; Merlini, L. J. Chem. Soc., Perkin Trans. 1 1995, 2447.
[22] Ishii, H.; Koyama, H.; Hagiwara, K.; Miura, T.; Xue, G. G.; Hashimoto, Y.; Kitahara, G.; Aida, Y.; Suzuki, M. Bioorg. Med. Chem. Lett. 2012, 22, 1469.
[23] Javed, U.; Karim, M.; Jahng, K. C.; Park, J. G.; Jahng, Y. D. Tetrahedron: Asymmetry 2014, 25, 1270.
[24] Huang, Y. D.; Zhang, J. S.; Pettus, T. R. R. Org. Lett. 2005, 7, 5841.
[25] Lin, C. C.; Teng, T. M.; Tsai, C. C.; Liao, H. Y.; Liu, R. S. J. Am. Chem. Soc. 2008, 130, 16417.
[26] Pan, C. X.; Zeng, X. H.; Guan, Y. F.; Jiang, X. L.; Li, L.; Zhang, H. B. Synlett 2011, 425.
[27] Wang, X. Q.; Zhang, H. B.; Yang, X. D.; Zhao, J. F.; Pan, C. X. Chem. Commun. 2013, 49, 5405.
[28] Li, L. Q.; Li, M. M.; Wang, K.; Qin, H. B. Tetrahedron Lett. 2013, 54, 6029.
[29] Yadav, J. S.; Mishra, A. K.; Das, S. Tetrahedron 2014, 70, 7560.
[30] Jung, Y.; Kim, I. J. Org. Chem. 2015, 80, 2001.
/
| 〈 |
|
〉 |