

Chinese Journal of Organic Chemistry >
Transmembrane Transport of Actylcholine through Artificial Channels
Received date: 2015-05-03
Revised date: 2015-05-13
Online published: 2015-05-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21422202).
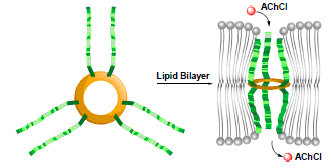
In natural system, the transmembrane transport of actylcholine is one of the important processes for realizing the neural signal transduction, which relies on some specific proteins. The development of artificial systems displaying the similar transport capability is not only fundamentally important but also may find potential biological and medical applications. However, this has been a challenge because of lacking architectures with proper cavity size for passing of actylcholine. In this paper, the artificial channels have been built from the Phe tripeptide attached to aromatic hydrazide macrocycles. Dynamic fluorescent experiments on lipid vesicles and patch clamp experiments on planar lipid bilayer revealed that the channels can transport actylcholine. The transport selectivity was found to increase with the increasing cavity size of the macrocycles.
Zhu Pingping , Xin Pengyang , Hou Jun-Li , Li Zhan-Ting . Transmembrane Transport of Actylcholine through Artificial Channels[J]. Chinese Journal of Organic Chemistry, 2015 , 35(9) : 1994 -1998 . DOI: 10.6023/cjoc201505003
[1] Yang, F.-Y. Biomembranes, Science Press, Beijing, 2005 (in Chinese). (杨福愉, 生物膜, 科学出版社, 北京, 2005.)
[2] Hille, B. Ionic Channels of Excitable Membranes, 3rd ed, Sinauer Associates, Sunderland, MA, 2001.
[3] Ashcroft, F. M. Ion Channels and Disease, Academic Press, San Diego and London, 2000.
[4] (a) Sakai, N.; Mareda, J.; Matile, S. Acc. Chem. Res. 2005, 38, 79. (b) Li, X.; Wu, Y.-D.; Yang, D. Acc. Chem. Res. 2008, 41, 1428. (c) Legrand, Y.-M.; Barboiu, M. Chem. Rec. 2013, 13, 524. (d) Matle, S.; Fyles, T. M. Acc. Chem. Res. 2013, 46, 2741. (e) Gong, B.; Shao, Z. Acc. Chem. Res. 2013, 46, 2856. (f) Montenegro, J.; Ghadiri, M. R.; Granja, J. R. Acc. Chem. Res. 2013, 46, 2955. (g) Gokel, G. W.; Murillo, O. Acc. Chem. Res. 1996, 29, 425. (h) Mayer, M.; Yang, J. Acc. Chem. Res. 2013, 46, 2998. (i) Xin, P.; Zhu, P.; Su, P.; Hou, J.-L.; Li, Z.-T. J. Am. Chem. Soc. 2014, 136, 13078.
[5] (a) Cho, H.; Widanapathirana, L.; Zhao, Y. J. Am. Chem. Soc. 2011, 133, 141. (b) Hu, X.-B.; Chen, Z.; Tang, G.; Hou, J.-L.; Li, Z.-T. J. Am. Chem. Soc. 2012, 134, 8384. (c) Chen, L.; Si, W.; Zhang, L.; Tang, G.; Li, Z.-T.; Hou, J.-L. J. Am. Chem. Soc. 2013, 135, 2152.
[6] (a) Parsons, S. M.; Prior, C.; Marshall, I. G. Int. Rev. Neurobiol. 1993, 35, 279. (b) Usdin, T. B.; Eiden, L. E.; Bonner, T. I.; Erickson, J. D. Trends Neurosci. 1995, 18, 218.
[7] Xin, P.; Zhang, L.; Su, P.; Hou, J.-L.; Li, Z.-T. Chem. Commun. 2015, 51, 4819.
[8] Busschaert, N.; Wenzel, M.; Light, M. E.; Iglesias-Hernandez, P.; Perez-Tomas, R.; Gale, P. A. J. Am. Chem. Soc. 2011, 133, 14136.
[9] Matile, S.; Jentzsch, A. V.; Montenegro, J.; Fin, A. Chem. Soc. Rev. 2011, 40, 2453.
[10] Chui, J. K. W.; Fyles, T. M. Chem. Soc. Rev. 2012, 41, 148.
[11] Fyles, T. M.; Loock, D.; van Straaten-Nijenhuis, W. F.; Zhou, X. J. Org. Chem. 1996, 61, 8866.
[12] Jeon, Y. J.; Kim, H.; Jon, S.; Selvapalam, N.; Oh, D. Y.; Seo, I.; Park, C. S.; Jung, S. R.; Koh, D. S.; Kim, K. J. Am. Chem. Soc. 2004, 126, 15944.
[13] Ashley, R. H. In Ion Channels: a Practical Approach, Ed.: Ashley, R. H., Oxford University Press, Oxford, UK, 1995.
/
| 〈 |
|
〉 |