

Chinese Journal of Organic Chemistry >
Imidazolinium Perrhenate-Catalyzed Deoxydehydration ofVicinal Diols to Alkenes
Received date: 2015-03-11
Revised date: 2015-05-14
Online published: 2015-05-29
Supported by
Project supported by the Natural Science Foundation of Heilongjiang Province (No. B200604), the Scientific Research Foundation for Returned Scholars, Ministry of Education of China, the Science and Technology Innovation Program of Harbin Science and Technology Bureau for Returned Scholars (No. 2007RFLXG016), the Foundation of Harbin Normal University (No. KG2007-05), the National Undergraduate Training Programs for Innovation and Entrepreneurship (No. 201310712087) and the Foundation of Northwest A&F University (No. Z111021007).
The deoxydehydration of polyols to alkenes is one of the important methods for the conversion of biomass-based platform molecules to useful chemicals and liquid fuels. A new imidazolinium perrhenate catalytic system for the deoxydehydration of vicinal diols to alkenes was reported. The reaction conditions, including reductants, solvents and imidazolinium cations were investigated. The best result was obtained with 3 equiv. of 3-octanol as the reductant in chlorobenzene at 180 ℃ under argon atmosphere, affording the 1-tetradecene product in 85% yield after 24 h. The catalyst shows moderate activity for several vicinal diols and can be reused after recoverd.
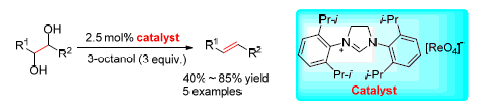
Key words: rhenium catalyst; vicinal diols; deoxydehydration; alkenes; imidazolinium salt
Sun Huimin , Hu Chen , Hao Zhiming , Zuo Yajie , Wang Tianchi , Zhong Chongmin . Imidazolinium Perrhenate-Catalyzed Deoxydehydration ofVicinal Diols to Alkenes[J]. Chinese Journal of Organic Chemistry, 2015 , 35(9) : 1904 -1909 . DOI: 10.6023/cjoc201503015
[1] (a) Huber, G. W.; Iborra, S.; Corma, A. Chem. Rev. 2006, 106, 4044.(b) Chheda, J. N.; Huber, G. W.; Dumesic, J. A. Angew. Chem., Int. Ed. 2007, 46, 7164.(c) Alonso, D. M.; Bond, J. Q.; Dumesic, J. A. Green Chem. 2010, 12, 1493.(d) Gallezot, P. Chem. Soc. Rev. 2012, 41, 1538.(e) Zhang, J. R.; Deng, T. C.; Liu, H. C. Prog. Chem. 2013, 25 (in Chinese).(张家仁, 邓甜超, 刘海超, 化学进展, 2013, 25.)(f) Yang, Z.; Fu, Y,; Guo, Q. Chin. J. Org. Chem. 2015, 35, 273 (in Chinese).(杨珍, 傅尧, 郭庆祥, 有机化学, 2015, 35, 273.)(g) Li, J.; Huang, Y.; Guo, Q.; Fu, Y. Acta Chim. Sin. 2014, 72, 1223 (in Chinese).(李江, 黄耀兵, 郭庆祥, 傅尧, 化学学报, 2014, 72, 1223.)(h) Lian, Y.; Yan, L.; Wang, Y.; Qi, X. Acta Chim. Sin. 2014, 72, 502 (in Chinese).(廉优芬, 闫碌碌, 王羽, 漆新华, 化学学报, 2014, 72, 502.)
[2] (a) Mohan, D.; Pittman, C. U.; Steele, P. H. Energy Fuels 2006, 20, 848.(b) Zhang, Q.; Chang,; Wang, T. J.; Xu, Y. Energy Convers. Manage. 2007, 48, 87.
[3] (a) Mascal, M. E.; Nikitin, B. Green Chem. 2010, 12, 370.(b) Jin, F.; Enomoto, H. Energy Environ. Sci. 2011, 4, 382.(c) Yang, W.; Grochowski, M. R.; Sen, A. ChemSusChem 2012, 5, 1218.
[4] (a) Corma, A.; Iborra, S.; Velty, A. Chem. Rev. 2007, 107, 2411.(b) Schlaf, M.; Ghosh, P.; Fagan, P. J.; Hauptman, E.; Bullock, R. M. Adv. Synth. Catal. 2009, 351, 78.
[5] Dethlefsen, J. R.; Fristrup, P. ChemSusChem 2015, 8, 767.
[6] Cook, G. K.; Andrews, M. A. J. Am. Chem. Soc. 1996, 118, 9448.
[7] Ziegler, J. E.; Zdilla, M. J.; Evans, A. J.; Abu-Omar, M. M. Inorg. Chem. 2009, 48, 9998.
[8] Vkuturi, S.; Chapman, G.; Ahmad, I.; Nicholas, K. M. Inorg. Chem. 2010, 49, 4744.
[9] Arceo, E.; Ellman, J. A.; Bergman, R. G. J. Am. Chem. Soc. 2010, 132, 11408.
[10] Shiramizu, M.; Toste, F. D. Angew. Chem. Int. Ed. 2012, 51, 8082.
[11] McClain, J. M.; Nicholas, K. M. ACS Catal. 2014, 4, 2109.
[12] Bi, S.; Wang, J.; Liu, L.; Li, P.; Lin, Z. Organometallics 2012, 31, 6139.
[13] Mao, G. L.; Jia, B.; Wang, C. Y. Chin. J. Org. Chem. 2014, 34, 32 (in Chinese).(毛国梁, 贾冰, 王从洋, 有机化学, 2014, 34, 32.)
[14] Ahmad, I.; Chapman, G.; Nicholas, K. M. Organometallics 2011, 30, 2810.
[15] Sousa, S. C.; Fernandes, A. C. Tetrahedron Lett. 2011, 52, 6960.
[16] Yi, J.; Liu, S.; Abu-Omar, M. M. ChemSusChem 2012, 5, 1401.
[17] Boucher-Jacobs, C.; Nicholas, K. M. ChemSusChem 2013, 6, 597.
[18] Raju, S.; Jastrzebski, J. T. B. H.; Lutz, M.; Klein Gebbink, R. J. M. ChemSusChem 2013, 6, 1673.
[19] Denning, A. L.; Dang, H.; Liu, Z. M.; Nicholas, K. M.; Jentoft, F. C. ChemCatChem 2013, 12, 3567.
[20] Shiramizu, M; Toste, F. D. Angew. Chem., Int. Ed. 2013, 52, 12905.
[21] (a) Hills, L.; Moyano, R.; Montilla, F.; Pastor, A.; Galindo, A.; Alvarez, E.; Marchetti, F.; Pettinari, C. Eur. J. Inorg. Chem. 2013, 3352.(b) Dethlefsen, J. R.; Lupp, D.; Oh, B, C.; Fristrup P. ChemSusChem 2014, 7, 425.
[22] (a) Murru, S.; Nicholas, K. M.; Srivastava, R. S. J. Mol. Catal. A. Chem. 2012, 363, 460.
[23] (b) Stanowski, S.; Nicholas, K. M.; Srivastava, R. S. Organometallics 2012, 31, 515.
[24] Nicholas, K. M.; Chapman, G. Chem. Commun. 2013, 49, 8199.
[25] (a) Sasaki, T.; Zhong, C.; Tada, M.; Iwasawa, Y. Chem. Commun. 2005, 2506.(b) Zhong, C.; Sasaki, T.; Tada, M.; Iwasawa, Y. Catal. J. 2006, 242, 357.(c) Zhong, C.; Sasaki, T.; Jimbo-Kobayashi, A.; Fujiwara, E.; Kobayashi, A.; Tada, M.; Iwasawa, Y. Bull. Chem. Soc. Jpn. 2007, 80, 2365.(d) Sasaki, T.; Tada, M.; Zhong, C.; Kume, T.; Iwasawa, Y. J. Mol. Catal. A: Chem. 2008, 279, 200.
[26] The molar ratio of imidazolinium cation to water molecule in the crystal is 2:1, and the water molecule is the disorder state.
[27] Thomson, J. E.; Campbell, C. D.; Concello′n, C.; Duguet, N.; Rix, K.; Slawin, A. M. Z.; D. Smith, A. J. Org. Chem. 2008, 73, 2784.
/
| 〈 |
|
〉 |