

Chinese Journal of Organic Chemistry >
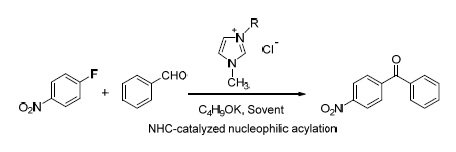
Nucleophilic Acylation of 4-Fluoronitrobenzene with Benzaldehyde Catalyzed by N-Heterocyclic Carbene
Received date: 2015-03-24
Revised date: 2015-05-26
Online published: 2015-06-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21006055) and the Beijing National Laboratory for Molecular Sciences (No. 20140141).
Imidazolium ionic liquids as the catalyst precursor of N-heterocyclic carbene (NHC) have been applied to catalyze the nucleophilic acylation of arylfluoride with aromatic aldehyde or aliphatic aldehyde. The acyl group can be directly introduced to the aromatic ring with an electron-withdrawing group. The reaction of 4-fluoronitrobenzene with benzaldehyde for the preparation of 4-nitrobenzophenone is selected as the model reaction. The mechanism of nucleophilic acylation catalyzed by NHC is proposed. The ionic liquid firstly is converted to NHC under the presence of alkali. NHC makes benzaldehyde carry through the umpolung, and the acyl of benzaldehyde as the nucleophilic group attacks the carbon atom bearing fluorine of 4-fluoronitrobenzene, followed by loss of the fluorine as an anion. Base-promoted eliminations of a proton and NHC from the intermediate take place to afford the target product of 4-nitrobenzophenone. The effects of the alkyl chain of the ionic liquid, the amount of ionic liquid, different solvent, the type of alkali, the amount of alkali, reaction temperature and reaction time on the catalytic reaction have been fully investigated. The selected optimal conditions are as follows: DMSO as the solvent, the ionic liquid 1 (R=n-C12H25) as the catalyst precursor, potassium tert-butoxide as the alkali, the amount of the ionic liquid 33 mol%, the molar ratio of the ionic liquid to the alkali 1:4, reaction temperature 30 ℃ and reaction time 6 h. Under the above optimal conditions, the conversion of 4-fluoronitrobenzene is 93.0%, and the yield of 4-nitrobenzophenone is 88.6%. After the reaction, the ionic liquid is dried under vacuum and then can be directly reused. The recycling efficiency of the ionic liquid is investigated. The result showed that the yield of 4-nitrobenzophenone still could reach above 75% after 4 recycles of the ionic liquid. The study provides a new environmentally-friendly method for the direct introduction of the acyl group to the electron-deficient aromatic ring to synthesize the aromatic ketones with electron-withdrawing group.
Yu Fengli , Lu Yuqian , Yuan Bing , Xie Congxia , Yu Shitao . Nucleophilic Acylation of 4-Fluoronitrobenzene with Benzaldehyde Catalyzed by N-Heterocyclic Carbene[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2223 -2228 . DOI: 10.6023/cjoc201503038
[1] Wöhler, F.; Liebig, J. Ann. Pharm. 1832, 3, 249.
[2] Lapworth, A. J. J. Chem. Soc. 1903, 83, 995.
[3] Ukai, T.; Tanaka, R.; Dokawa, T. J. Pharm. Soc. Jpn. 1943, 63, 296.
[4] Arduengo, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
[5] Yu, H.-Z.; Fu, Y.; Liu, L.; Guo, Q.-X. Chin. J. Org. Chem. 2007, 27, 545 (in Chinese).(于海珠, 傅尧, 刘磊, 郭庆祥, 有机化学, 2007, 27, 545.)
[6] Sun, X.-Y.; Wu, J. Chin. J. Org. Chem. 2006, 26, 745 (in Chinese).(孙小宇, 吴劼, 有机化学, 2006, 26, 745.)
[7] Qu, M.-N.; He, J.-M. Chin. J. Org. Chem. 2011, 31, 1388 (in Chinese).(屈孟男, 何金梅, 有机化学, 2011, 31, 1388.)
[8] Ca, X.-H.; Xie, B. Chin. J. Appl. Chem. 2013, 30, 123 (in Chinese).(蔡下华, 谢兵, 应用化学, 2013, 30, 123.)
[9] Bugant, X.; Glorius, F. Chem. Soc. Rev. 2012, 41, 3511.
[10] Chai, H. X.; Li, J. R.; Yang, L. P.; Liu, M. X.; Yang, D. L.; Zhang, Q.; Shi, D. X. Chin. J. Chem. 2014, 32, 865.
[11] Enders, D.; Kallfass, U. Angew. Chem., Int. Ed. 2002, 41, 1743.
[12] Mennen, S. M.; Gipson, J. D.; Kim, Y. R.; Miller, S. J. J. Am. Chem. Soc. 2005, 127, 1654.
[13] Enders, D.; Niemeier, O.; Balensiefer, T. Angew. Chem., Int. Ed. 2006, 45, 1463.
[14] Liu, Q.; Rovis, T. J. Am. Chem. Soc. 2006, 128, 2552.
[15] Li, Y.; Shi, F. Q.; He Q. L.; You, S. L. Org. Lett. 2009, 11, 3182.
[16] Xie, Y.-M.; Wu, J.; Chao, X.-J. Chem. Bioeng. 2011, 28, 10 (in Chinese).(谢益明, 吴杰, 晁雪静, 化学与生物工程, 2011, 28, 10.)
[17] He, M., Gerson, J. U.; Jeffrey, W. B. J. Am. Chem. Soc. 2006, 128, 15088.
[18] Yu, F. L.; Jiang, J. J.; Zhao, D. M.; Xie, C. X.; Yu, S. T. RSC Adv. 2013, 3, 3996.
[19] Yu, F.-L.; Dou, Y.-T.; Zhao, D.-M.; Xie, C.-X.; Yu, S.-T. Chin. J. Org. Chem. 2014, 34, 1190 (in Chinese).(于凤丽, 窦有涛, 赵冬梅, 解从霞, 于世涛, 有机化学, 2014, 34, 1190.)
[20] Yu, F.-L.; Zhao, D.-M.; Xie, C.-X.; Yu, S.-T. Chemistry 2014, 76, 712 (in Chinese).(于凤丽, 赵冬梅, 解从霞, 于世涛, 化学通报, 2014, 76, 712.)
[21] Grasa, G. A.; Kissling, R. M.; Nolan, S. P. Org. Lett. 2002, 4, 3583.
[22] Suzuki, Y.; Yamauchi, K.; Muramatsu, K.; Sato, M. Chem. Commun. 2004, 2770.
[23] Kano, T.; Sasaki, K.; Maruoka, K. Org. Lett. 2005, 7, 1347.
[24] Suzuki, Y.; Toyota, T.; Imada, F.; Satoa, M.; Miyashita, A. Chem. Commun. 2003, 1314.
[25] Suzuki, Y.; Ota, S.; Fukuta, Y.; Ueda, Y.; Sato, M. J. Org. Chem. 2008, 73, 2420.
[26] Suzuki, Y.; Toyota, T.; Miyashita, A.; Sato, M. Chem. Pharm. Bull. 2006, 54, 1653.
/
| 〈 |
|
〉 |