

Chinese Journal of Organic Chemistry >
Synthesis and Fungicidal Activity of a Series of Fluorinated Quinoline Amide Compounds
Received date: 2015-04-18
Revised date: 2015-06-03
Online published: 2015-06-12
Supported by
Project supported by the National Key Technologies R&D Program (No. 2011BAE06B02-04).
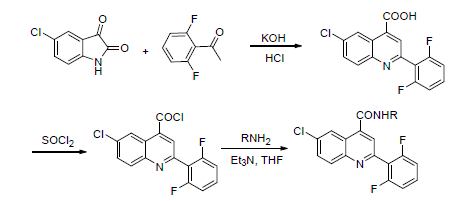
To find new fungicidal lead compounds with high bioactivity, novel lead compounds were designed by active group splicing. The synthetic routes are as follows: 6-chloro-2-(2,6-difluorophenyl)quinoline-4-carbonyl chloride (7) was synthesized using 5-chlorine isatin (4) and 2,6-difluoro acetophenone (5) as raw materials by cyclization, acyl chlorination and other step reactions. Then 13 novel title compounds were synthesized by acylation of 6-chloro-2-(2,6-difluorophenyl)-quino-line-4-carbonyl chloride (7) with substituted anilines (8). The structures of the compounds were confirmed by HRMS and 1H NMR. The preliminary fungicidal activity showed that all the compounds exhibit fungicidal activity against Gaeumannomyces graminis and Fusahum graminearum sehw, Rhizoctonia cerealis and Pyricularia grisea at concentration of 50 mg/L, part of compounds against Gaeumannomyces graminis were more than 90% at concentration of 50 mg/L.
Key words: fluorinated quinoline; amide compounds; synthesis; fungicidal activity
Ni Yun , Xu Tianming , Zhong Liangkun , Kong Xiaoyan , Shi Jianjun , Liu Xinghai , Kong Xiaolin , Ji Wenjuan , Tan Chengxia . Synthesis and Fungicidal Activity of a Series of Fluorinated Quinoline Amide Compounds[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2218 -2222 . DOI: 10.6023/cjoc201504025
[1] Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage Sci. 2015, 71, 292.
[2] Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84, 342.
[3] Nicholson, A. N.; Stone, B. M.; Clarke C. H. J. Clin. Pharmacol. 1977, 4, 567.
[4] Zayed, M. E. M.; El-Shishtawy, R. M.; Elroby, S. A.; Obaid, A. Y.; Al-amshany, Z. M. Int. J. Mol. Sci. 2015, 16, 3804.
[5] Wang, L.; ?witalska, M.; Wang, N.; Du, Z.-J.; Fukumoto, Y.; Diep, N. K.; Kiguchi, R.; Nokami, J.; Wietrzyk, J.; Inokuchi, T. Molecules 2014, 19, 19021.
[6] Fragoulis, G.; Merli, A.; Reeves, G.; Meregalli, G.; Stenberg, K.; Tanaka, T.; Capri, E. Pest Manage. Sci. 2011, 67, 656.
[7] Liu, C. L. World Pesticide: Fungicide, Chemical Industry Press, Beijing, 2006, p. 251 (in Chinese). (刘长令, 世界农药大全杀菌剂卷, 化学工业出版社, 北京, 2006, p. 251.)
[8] Nakano, H.; Masumizu, T. JP 2009091320, 2009 [Chem. Abstr. 2010, 150, 472698].
[9] Hackler, R. E.; Jourdan G. P.; Johnson, P. L.; Thoreen, B. R.; Samaritoni, J. G. WO 9304580, 1993 [Chem. Abstr. 1993, 119, 139111].
[10] (a) Liu, X. H.; Weng, J. Q.; Wang, B. L.; Li, Y. H.; Tan, C. X.; Li, Z. M. Chem. Res. Intermed. 2014, 40, 2605. (b) Yang, M. Y.; Zhao, W.; Sun, Z. H.; Tan, C. X.; Weng, J. Q.; Liu, X. H. Lett. Drug Des. Discovery 2015, 12, 314. (c) Xie, F.; Liu, T. T.; Yang, G.; Yuan, J.; Kong, X. L.; Xu, T. M.; Tan, C. X. Chin. J. Org. Chem. 2013, 33, 2596 (in Chinese). (谢峰, 刘婷婷, 杨果, 袁静, 孔小林, 许天明, 谭成侠, 有机化学, 2013, 33, 2596.)(d) Yan, S. L.; Yang, M. Y.; Sun, Z. H.; Min, L. J.; Tan, C. X.; Weng, J. Q.; Wu, H. K.; Liu, X. H. Lett. Drug Des. Discov. 2014, 11, 940. (e) Sun, G.-X.; Sun, Z.-H.; Yang, M.-Y.; Liu, X.-H.; Ma, Y.; Wei, Y.-Y. Molecules 2013, 18, 14876.
[11] Karen, L.; Daniel, D. S. Synthesis 1993, 993.
[12] Yu, M. Q.; Liu, G. Y.; Y, X. R.; Chen, Q. Y.; Di, N. Fine Chem. Intermed. 2011, 41, 1 (in Chinese). (喻名强, 刘广义, 伊兴荣, 陈秦豫, 狄宁, 精细化工中间体, 2011, 41, 1.)
[13] (a) Liu, X. H.; Pan, L.; Tan, C. X.; Weng, J. Q.; Wang, B. L.; Li, Z. M. Pest. Biochem. Physiol. 2011, 101, 143. (b) Wu, R.; Zhu, C.; Du, X. J.; Xiong, L. X.; Yu, S. J.; Liu, X. H.; Li, Z. M.; Zhao, W. G. Chem. Cent. J. 2012, 6, 99.
[14] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 558.
/
| 〈 |
|
〉 |