

Chinese Journal of Organic Chemistry >
Photocatalysis Synthesis and Cytotoxicity of α-Santonin Rearrangement Derivatives
Received date: 2015-05-11
Revised date: 2015-06-08
Online published: 2015-06-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372056, 21202027).
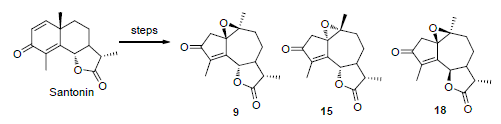
20 guaianolide derivatives 1~20 and 6-epi-santonin were synthesized from α-santonin through rearrangement, oxidation, and reduction, hydrolysis. The structures of all compounds were characterized by 1H NMR, 13C NMR and MS. The cell proliferation inhibiting activities of the target compounds were evaluated against A549 and HepG2 cell lines by thiazolyl blueterazolium bromide (MTT) method. The results showed that the epoxy guaiane 9 had obvious inhibitory effect on A549 and HepG2 cell lines with IC50 values of (14.5±0.1), (10.6±0.3) μmol/L while epoxy guaiane 15 showed IC50 values of (6.4±1.3) and (8.1±0.7) μmol/L.
Key words: α-santonin; guaiane; derivative; cytotoxicity
Dai Xiaoli , Tang Jian , Zhang Lingqiong , Tao Lianzhi , Ouyang Zhen , Wang Min . Photocatalysis Synthesis and Cytotoxicity of α-Santonin Rearrangement Derivatives[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2142 -2149 . DOI: 10.6023/cjoc201505020
[1] Wu, L. J. Textbook of Medicinal Chemistry of Natural Products, People's Medical Publishing House, Beijing, 2007, pp. 227~232 (in Chinese). (吴立军, 天然药物化学教材, 人民卫生出版社, 北京, 2007, pp. 227~232.)
[2] Saxena, V. K.; Mondal, S. K. Phytochemistry 1994, 35, 1080.
[3] Qian, M. K.; Zhu, D. Y. Pharm. Bull. 1979, 17, 277 (in Chinese ). (钱名堃, 朱大元, 药学通报, 1979, 17, 277.)
[4] Bruno, M.; Rosselli, S.; Maggio, A.; Raccuglia, R. A.; Bastow, K. F.; Lee, K. H. J. Nat. Prod. 2005, 68, 1042.
[5] René, C.; Anke, H.; Bianka, S.; Stefan, S.; Alexander, B.; Ralph, K.; Dieter, S. Pharm. Chem. Life Sci. 2012, 345, 215.
[6] Francisco, A. M.; Alejandro, S.; Azusa, Y.; Rosa, M., V.; Frank, R. F.; José, M. G. M. J. Nat. Prod. 2012, 75, 1967.
[7] Park, K. H.; Yang, M. S.; Park, M. K.; Kim, S. C.; Yang, C. H.; Park, S. J.; Lee, J. R. Fitoterapia 2009, 80, 54.
[8] Usuki, T.; Sato, M.; Hara, S.; Yoshimoto, Y.; Kondo, R.; Zimmermann, S.; Kaiser, M.; Brun, R.; Hamburger, M.; Adams, M. Bioorg. Med. Chem. Lett. 2014, 24, 794.
[9] Zhang, W. H.; Luo, S. J.; Fang, F.; Chen, C. S.; Hu, H. W.; Jia, X. S.; Zhai, H. B. J. Am. Chem. Soc. 2005, 127, 18.
[10] Li, C.; Dong, T.; Li, Q.; Lei, X. Angew. Chem., Int. Ed. 2014, 53, 12111.
[11] Li, C.; Yu, X. L.; Lei, X. G. Org. Lett. 2010, 12, 4284.
[12] Xia, W.-J.; Sun, L.-D.; Shi, L.; Zhang, S.-Y.; Tu, Y.-Q. Chin. J. Chem. 2004, 22, 377.
[13] Li, D. R.; Xia, W. J.; Shi, L.; Tu, Y. Q. Synthesis 2003, 41.
[14] Xia, W. J.; Tu, Y. Q.; Li, D. R. Synth. Commun. 2001, 31, 1613.
[15] Xia, D.; Du Y.; Yi, Z.; Song, H.; Qin, Y. Chem.-Eur. J. 2013, 19, 4423.
[16] Bargues, V.; Blay, G.; Cardona, L.; Garcia, B.; Pedro, J. R. Tetrahedron 1998, 54, 1845.
[17] Arantes, F. F. P.; Barbosa, L. C. A.; Alvarenga, E. S.; Demuner, A. J.; Bezerra, D. P.; Ferreira, J. R. O.; Costa-Lotufo, L. V.; Pessoa, C.; Moraes, M. O. Eur. J. Med. Chem. 2009, 44, 3739.
[18] Andrew, E. G.; Mark, T. E. J. Org. Chem. 1989, 54, 1468.
[19] Peter, M.; Simon, B.; Roland, F. J. Am. Chem. Soc. 1993, 115, 12595.
[20] Blay, G.; Cardona, L.; Garcia, B.; Lahoz, L.; Pedro, J. R. J. Org. Chem. 2001, 66, 7700.
[21] Makiyi, E. F.; Frade, R. F. M.; Lebl, T.; Jaffray, E. G.; Cobb, S. E.; Harvey, A. L.; Slawin, A. M. Z.; Hay, R. T.; Westwood, N. J. Eur. J. Org. Chem. 2009, 5711.
[22] Ando, M.; Akahane, A.; Yamaoka, H.; Takase, K. J. Org. Chem. 1982, 47, 3909.
/
| 〈 |
|
〉 |