

Chinese Journal of Organic Chemistry >
Design, Synthesis and Anion Recognition of Benzocoumarin-Based Thiourea Receptors
Received date: 2015-05-26
Revised date: 2015-06-12
Online published: 2015-06-19
Supported by
Project supported by the National Natural Science Foundation of China (No. J1103303), the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences (No. 20120010).
Anions display a great significant role in the environment and life science. The design and synthesis of receptors with high selective recognition anion has attracted considerable attention. The selective identification fluoride anion of benzocoumarin-based thiourea receptors 4a~4e were obtained starting from β-naphthol by a multi-step reaction. Their structures were characterized by 1H NMR, 13C NMR, IR and elemental analysis. The interactions between receptors 4a~4e and anions (F-, AcO-, C1-, Br-, I-, HSO4-) were studied by optical (UV-Vis absorption and fluorescence) and 1H NMR spectroscopy. The results showed that the receptors with anion through hydrogen bonds formed stable 1:2 complexes, which leaded to the change spectroscopy of receptor molecules. Determination of the combined ratio and the stability constants of complexes were borne out receptor molecules with high alternative recognition F- or AcO- anions with limit of detect of 5.45 and 7.00 μg/L and the effect of reaction time of 5 min and 10 min respectively. However, there is no selectively responds to other anions. Using NMR titration and theoretical (B3LYP/6-31G (d, p)) methods further confirmed the hydrogen bond acceptor molecules and anions.
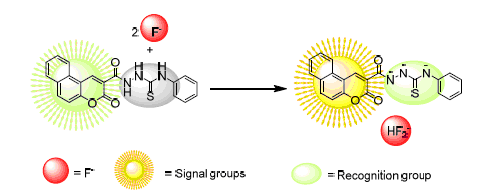
Key words: benzo coumarin; thiourea; anion recognition; hydrogen bond
Yan Xiquan , Zhuo Jibin , Wang Jicheng , Xie Lili , Yuan Yaofeng . Design, Synthesis and Anion Recognition of Benzocoumarin-Based Thiourea Receptors[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2184 -2190 . DOI: 10.6023/cjoc201505038
[1] Jiang, X. Z.; Feng, M. Y.; Zhang, D. W.; Wang, B. S.; Dong, Z. Y.; Gao, G. H. Chin. J. Chem. 2013, 31, 673.
[2] Yang, Z. P.; Zhang, K.; Gong, F. B.; Li, S. Y.; Chen, J.; Ma, J. S.; Sobenina, L. N.; Mikhaleva, A. I.; Trofimov, B. A.; Yang, G. Q. J. Photochem. Photobiol., A 2011, 217, 29.
[3] Liu, R. Y.; Yong, X.; Yang, X. D.; Yan, Y. S.; Lu, X. W.; Qu, J. Q. Chin. J. Org. Chem. 2014, 34, 561 (in Chinese).(刘瑞源, 勇雪, 杨小东, 严轶琛, 路新卫, 瞿金清, 有机化学, 2014, 34, 561.)
[4] Chen, H. M.; Yue, F.; Lin, H. K. Chem. J. Chin. Univ. 2012, 33(6), 1239 (in Chinese).(陈华梅, 岳凡, 林华宽, 高等学校化学学报, 2012, 33(6), 1239.)
[5] Zhang, Y. M.; Wang, A. X.; Cao, C.;Leng, Y. L.; Wei, T. B. Chin. J. Chem. 2009, 27, 1617.
[6] Çak?l, E. Ind. Eng. Chem. Res. 2000, 39(10), 3582.
[7] Brunet, E.; García-Losada, P.; Rodríguez-Ubis, J. C.; Juanes, O. Can. J. Chem. 2002, 80(2), 169.
[8] Peng, M. S.; Cai, J. Dyes Pigm. 2008, 79(3), 270.
[9] Maity, D.; Govindaraju, T. Inorg. Chem. 2010, 49(16), 7229.
[10] Zhang, Q.; Zhu, Z.; Zheng, Y.; Zheng, Y. L.; Cheng, J. G.; Zhang, N.; Long, Y. T.; Zheng, J.; Qian, X. H.; Yang, Y. J. J. Am. Chem. Soc. 2012, 134(45), 18479.
[11] Zhou, Y.; Zhang, J. F.; Yoon, J. Chem. Rev. 2014, 114(10), 5511.
[12] Yadav, U. N.; Pant, P.; Sharma, D.; Sahoo, S. K.; Shankarling, G. S. Sens. Actuators, B 2014, 197, 73.
[13] Qian, L. J.; Tong, B.; Shen, J. B.; Shi, J. B.; Zhi, J. G.; Dong, Y. Q.; Yang, F.; Dong, Y. P.; Lam, J. W. Y.; Liu, Y.; Tang, B. Z. Phys. Chem. B 2009, 113, 9089
[14] Pocker, Y.; Ciula, J. C. J. Am. Chem. Soc. 1989, 111(13), 4728.
[15] Li, S. W.; Cao, X. F.; Chen, C. S.; Ke, S. Y. Spectrochim. Acta, Part A 2012, 96, 18.
[16] Zhang, H.; Yu, T. Z.; Zhao, Y. L.; Fan, D. W.; Qian, L.; Yang, C. H.; Zhang, K. Spectrochim. Acta, Part A 2007, 68(3), 725.
[17] Maggi, R.; Bigi, F.; Carloni, S.; Mazzacani, A.; Sartori, G. Green Chem. 2001, 3(4), 173.
/
| 〈 |
|
〉 |