

Chinese Journal of Organic Chemistry >
Asymmetric Synthesis of Spirooxindoles via Organocascade Strategies
Received date: 2015-04-28
Revised date: 2015-06-16
Online published: 2015-07-02
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372235).
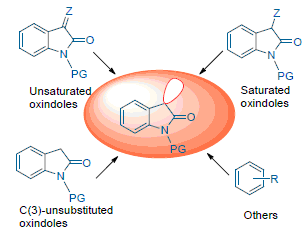
The spirooxindoles have broad and promising activities in various therapeutic areas, and become a privileged skeleton for drug discovery. Therefore, the development of some simple and efficient strategies to build these sophisticated scaffolds has become one of the most widespread concerns. However, the traditional methods are limited by the separation and purification of intermediates, the functional group protection and de-protection. Recently, the cascade strategies have shown special advantages in the synthesis of optically active natural products and complex molecules, many related studies have been reported. This review summarizes the enantioselective synthesis of spirooxindoles via cascade strategies in the past five years and organized on the basis of four types of starting materials: unsaturated oxindole derivatives, C(3)-substituted oxindoles, C(3)-unsubstituted oxindoles and nonoxindoles.
Key words: spirooxindoles; asymmetric; catalysis; organocascade
Xiao Yonglong , Zhou Yu , Wang Jiang , Wang Jinxin , Liu Hong . Asymmetric Synthesis of Spirooxindoles via Organocascade Strategies[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2035 -2048 . DOI: 10.6023/cjoc201504045
[1] Lin, H.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2003, 42, 36.
[2] Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Tomita, Y.; Parrish, D. A.; Deschamps, J. R.; Wang, S. J. Am. Chem. Soc. 2005, 127, 10130.
[3] Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R. S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; Bernard, D.; Zhang, J.; Lu, Y.; Gu, Q.; Shah, R. B.; Pienta, K. J.; Ling, X.; Kang, S.; Guo, M.; Sun, Y.; Yang, D.; Wang, S. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 3933.
[4] (a) Rottmann, M.; McNamara, C.; Yeung, B. K.; Lee, M. C.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D. M.; Dharia, N. V.; Tan, J.; Cohen, S. B.; Spencer, K. R.; Gonzalez-Paez, G. E.; Lakshminarayana, S. B.; Goh, A.; Suwanarusk, R.; Jegla, T.; Schmitt, E. K.; Beck, H. P.; Brun, R.; Nosten, F.; Renia, L.; Dartois, V.; Keller, T. H.; Fidock, D. A.; Winzeler, E. A.; Diagana, T. T. Science. 2010, 329, 1175.(b) Yeung, B. K.; Zou, B.; Rottmann, M.; Lakshminarayana, S. B.; Ang, S. H.; Leong, S. Y.; Tan, J.; Wong, J.; Keller-Maerki, S.; Fischli, C.; Goh, A.; Schmitt, E. K.; Krastel, P.; Francotte, E.; Kuhen, K.; Plouffe, D.; Henson, K.; Wagner, T.; Winzeler, E. A.; Petersen, F.; Brun, R.; Dartois, V.; Diagana, T. T.; Keller, T. H. J. Med. Chem. 2010, 53, 5155.
[5] Jiang, K.; Jia, Z. J.; Yin, X.; Wu, L.; Chen, Y. C. Org. Lett. 2010, 12, 2766.
[6] (a) Erkkila, A.; Majander, I.; Pihko, P. M. Chem. Rev. 2007, 107, 5416.(b) Melchiorre, P. Angew. Chem., Int. Ed. 2012, 51, 9748.(c) Mukherjee, S.; Yang, J. W.; Hoffmann, S.; List, B. Chem. Rev. 2007, 107, 5471.(d) Xu, L. W.; Luo, J.; Lu, Y. Chem. Commun. 2009, 1807.
[7] Bencivenni, G.; Wu, L. Y.; Mazzanti, A.; Giannichi, B.; Pesciaioli, F.; Song, M. P.; Bartoli, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2009, 48, 7200.
[8] Jiang, K.; Jia, Z. J.; Chen, S.; Wu, L.; Chen, Y. C. Chemistry 2010, 16, 2852.
[9] Liu, Y.; Nappi, M.; Arceo, E.; Vera, S.; Melchioree, P. J. Am. Chem. Soc. 2011, 133, 15212.
[10] Wang, G.; Liu, X.; Huang, T.; Kuang, Y.; Lin, L.; Feng, X. Org. Lett. 2013, 15, 76.
[11] Qi, L. W.; Yang, Y.; Gui, Y. Y.; Zhang, Y.; Chen, F.; Tian, F.; Peng, L.; Wang, L. X. Org. Lett. 2014, 16, 6436.
[12] Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713.
[13] Zhang, Z.; Schreiner, P. R. Chem. Soc. Rev. 2009, 38, 1187.
[14] Tan, B.; Hernandez-Torres, G.; Barbas, C. F. J. Am. Chem. Soc. 2011, 133, 12354.
[15] Mao, H.; Lin, A.; Tang, Y.; Shi, Y.; Hu, H.; Cheng, Y.; Zhu, C. Org. Lett. 2013, 15, 4062.
[16] Tan, B.; Candeias, N. R.; Barbas, C. F. J. Am. Chem. Soc. 2011, 133, 4672.
[17] Zhu, G.; Sun, W.; Wu, C.; Li, G.; Hong, L.; Wang, R. Org. Lett. 2013, 15, 4988.
[18] Zhao, Q. Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724.
[19] Voituriez, A.; Pinto, N.; Neel, M.; Retailleau, P.; Marinetti, A. Chemistry 2010, 16, 12541.
[20] Chen, X. H.; Wei, Q.; Luo, S. W.; Xiao, H.; Gong, L. Z. J. Am. Chem. Soc. 2009, 131, 13819.
[21] Li, G.; Liang, T.; Wojtas, L.; Antilla, J. C. Angew. Chem., Int. Ed. 2013, 52, 4628.
[22] Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Angew. Chem., Int. Ed. 2011, 50, 7837.
[23] Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Chem. Sci. 2012, 3, 1231.
[24] Suman, K.; Srinu, L.; Thennarasu, S. Org. Lett. 2014, 16, 3732.
[25] Badillo, J. J.; Ribeiro, C. J.; Olmstead, M. M.; Franz, A. K. Org. Lett. 2014, 16, 6270.
[26] Chen, X.; Chen, H.; Ji, X.; Jiang, H.; Yao, Z. J.; Liu, H. Org. Lett. 2013, 15, 1846.
[27] Xu, J.; Mou, C.; Zhu, T.; Song, B. A.; Chi, Y. R. Org. Lett. 2014, 16, 3272.
[28] Duce, S. P., F.; Gramigna, L.; Bernardi, L.; Mazzanti, A.; Ricci, A.; Bartoli, G.; Bencivenni, G. Adv. Synth. Catal. 2011, 353, 5.
[29] Badillo, J. J.; Silva-Garcia, A.; Shupe, B. H.; Fettinger, J. C.; Franz, A. K. Tetrahedron Lett. 2011, 52, 5550.
[30] Jiang, X.; Cao, Y.; Wang, Y.; Liu, L.; Shen, F.; Wang, R. J. Am. Chem. Soc. 2010, 132, 15328.
[31] Dugal-Tessier, J.; O'Bryan, E. A.; Schroeder, T. B.; Cohen, D. T.; Scheidt, K. A. Angew. Chem., Int. Ed. 2012, 51, 4963.
[32] Que, Y.; Li, T.; Yu, C.; Wang, X. S.; Yao, C. J. Org. Chem. 2015, 80, 3289.
[33] Li, J. L.; Sahoo, B.; Daniliuc, C. G.; Glorius, F. Angew. Chem., Int. Ed. 2014, 53, 10515.
[34] Chen, X. J.; Qian, W. K.; Feng, E. G.; Zhou, Y.; Wang, J. F.; Jiang, H. L.; Yao, Z. J.; Liu. H. Adv. Synth. Catal. 2012, 354, 6.
[35] Albertshofer, K.; Tan, B.; Barbas, C. F. Org. Lett. 2012, 14, 1834.
[36] Liu, Z. S.; Li, W. K.; Kang, T. R.; He, L.; Liu, Q. Z. Org. Lett. 2015, 17, 150.
[37] Wang, Y.; Liu, L.; Zhang, T.; Zhong, N. J.; Wang, D.; Chen, Y. J. J. Org. Chem. 2012, 77, 4143.
[38] Peng, J.; Huang, X.; Jiang, L.; Cui, H. L.; Chen, Y. C. Org. Lett. 2011, 13, 4584.
[39] Cai, H.; Zhou, Y.; Zhang, D.; Xu, J. Y.; Liu, H. Chem. Commun. 2014, 50, 14771.
[40] Sun, W.; Zhu, G.; Wu, C.; Li, G.; Hong, L.; Wang, R. Angew. Chem., Int. Ed. 2013, 52, 8633.
[41] Liu, X. L.; Han, W. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2013, 15, 1246.
[42] Bergonzini, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2012, 51, 971.
[43] McInturff, E. L.; Mowat, J.; Waldeck, A. R.; Krische, M. J. J. Am. Chem. Soc. 2013, 135, 17230.
[44] Companyo, X.; Zea, A.; Alba, A. N.; Mazzanti, A.; Moyano, A.; Rios, R. Chem. Commun. 2010, 46, 6953.
[45] Du, D.; Hu, Z.; Jin, J.; Lu, Y.; Tang, W.; Wang, B.; Lu, T. Org. Lett. 2012, 14, 1274.
[46] Zhao, S.; Lin, J. B.; Zhao, Y. Y.; Liang, Y. M.; Xu, P. F. Org. Lett. 2014, 16, 1802.
[47] Zhou, B.; Yang, Y.; Shi, J.; Luo, Z.; Li, Y. J. Org. Chem. 2013, 78, 2897.
[48] Dou, X.; Lu, Y. Chem.-Eur. J. 2012, 18, 8315.
[49] Cheng, M. N.; Wang, H.; Gong, L. Z. Org. Lett. 2011, 13, 2418.
[50] Wang, J.; Yuan, Y.; Xiong, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2012, 14, 2210.
[51] Wang, H.; Guo, L. N.; Duan, X. H. Org. Lett. 2013, 15, 5254.
[52] Sharma, N.; Li, Z.; Sharma, U. K.; Van der Eycken, E. V. Org. Lett. 2014, 16, 3884.
[53] Deppermann, N.; Thomanek, H.; Prenzel, A.; Maison, W. J. Org. Chem. 2010, 75, 5994.
/
| 〈 |
|
〉 |