

Chinese Journal of Organic Chemistry >
Progress in Platinum-Catalyzed Cycloaddition Reactions
Received date: 2015-05-15
Revised date: 2015-06-18
Online published: 2015-07-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172081, 21372090), the Natural Science Foundation of Guangdong Province (No. S2013020013091) and the City of Guangzhou Science and Technology Plan Projects (No. 156300018).
The recent progress in platinum-catalyzed cycloaddition reactions is reviewed, including [3+2], [4+2], [4+3], [2+2] and [2+1] cycloaddition reactions. Moreover, the possible mechanisms of some parts of cycloaddition reactions are discussed and the characteristics of the reactions are also presented.
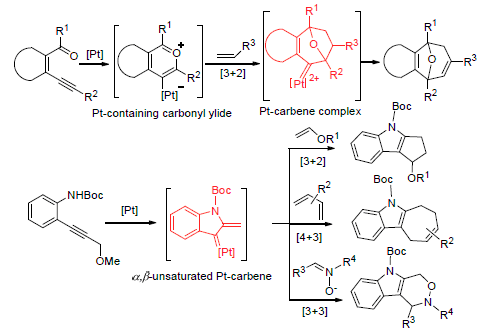
Key words: platinum catalysis; cycloaddition reactions; research progress
Cheng Guo , Yang Dingqiao . Progress in Platinum-Catalyzed Cycloaddition Reactions[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2023 -2034 . DOI: 10.6023/cjoc201505023
[1] Diels, O.; Alder, K. Justus Liebigs Ann. Chem. 1928, 460, 98.
[2] Luo, R.-S.; Yang, D.-Q. Chin. J. Org. Chem. 2007, 27, 958 (in Chinese). (罗人仕, 杨定乔, 有机化学, 2007, 27, 958.)
[3] Hu, P.; Long, Y.-H.; Wang, H.; Mo, H.-H. Chin. J. Org. Chem. 2008, 28, 1181 (in Chinese). (胡萍, 龙玉华, 王辉, 莫海洪, 杨定乔, 有机化学, 2008, 28, 1181.)
[4] Bian, H.-X.; Yang, D.-Q. Chin. J. Org. Chem. 2010, 30, 506 (in Chinese). (边红旭, 杨定乔, 有机化学, 2010, 30, 506.)
[5] Duan, Z.-B.; Long, Y.-H.; Yang, D.-Q. Chin. J. Org. Chem. 2010, 30, 368 (in Chinese). (段泽斌, 龙玉华, 杨定乔, 有机化学, 2010, 30, 368.)
[6] Zeng, Z.-Y.; Yang, D.-Q. Chin. J. Org. Chem. 2013, 33, 2131 (in Chinese). (曾中一, 杨定乔, 有机化学, 2013, 33, 2131.)
[7] (a) Kumar, K. R. R.; Mallesha, H.; Rangappa, K. S. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 159. (b) Kunz, R. K.; MacMillan, D. W. C. J. Am. Chem. Soc. 2005, 127, 3240. (c) Kumar, R. S.; Perumal, S.; Shetty, K. A.; Yogeeswari, P.; Sriram, D. Eur. J. Med. Chem. 2010, 45, 124. (d) Loh, B.; Vozzolo, L.; Mok, B. J.; Lee, C. C.; Fitzmaurice, J.; Caddick, S.; Fassati, A. Chem. Biol. Drug Des. 2010, 75, 461.
[8] (a) Suga, H.; Nakajima, T.; Itoh, K.; Kakehi, A. Org. Lett. 2005, 7, 1431. (b) Chen, D.-H.; Wang, Z.; Li, J.-T.; Yang, Z.-G.; Lin, L.-L.; Liu, X.-H.; Feng, X.-M. Chem. Eur. J. 2011, 17, 5226. (c) Suga, H.; Funyu, A.; Kakehi, A. Org. Lett. 2007, 9, 97.
[9] (a) Yudha, S.; Kuninobu, Y.; Takai, K. Angew. Chem., Int. Ed. 2008, 47, 9318. (b) Chaudhuri, R.; Liao, H.-Y.; Liu, R.-S. Chem. Eur. J. 2009, 15, 8895.
[10] Funami, H.; Kusama, H.; Iwasawa, N. Angew. Chem., Int. Ed. 2007, 46, 909.
[11] Kusama, H.; Ebisawa, M.; Funami, H.; Iwasawa, N. J. Am. Chem. Soc. 2009, 131, 16352.
[12] Kusama, H.; Miyashita, Y.; Takaya, J.; Iwasawa, N. Org. Lett. 2006, 8, 289.
[13] Jun, T.; Yui, C. M.; Hiroyuki, K.; Iwasawa, N. Tetrahedron 2011, 67, 4455.
[14] Kusama, H.; Funami, H.; Takaya, J.; Iwasawa, N. Org. Lett. 2004, 6, 605.
[15] Kusama, H.; Funami, H.; Iwasawa, N. Synthesis 2007, 2014.
[16] Kusama, H.; Ishida, K.; Funami, H.; Iwasawa, N. Angew. Chem., Int. Ed. 2008, 47, 4903.
[17] Ishida, K.; Kusama, H.; Iwasawa, N. J. Am. Chem. Soc. 2010, 132, 842.
[18] Kusama, H.; Watanabe, E.; Ishida, K.; Iwasawa, N. Chem. Asian J. 2011, 6, 2273.
[19] Oh, C. H.; Yi, H. J.; Lee, J. H.; Lim, D. H. Chem. Commun. 2010, 46, 3007.
[20] Kim, N.; Kim, Y.; Park, W.; Sung, D.; Gupta, A. K.; Oh, C. H. Org. Lett. 2005, 7, 5289.
[21] Shin, S.; Gupta, A. K.; Rhim, C. Y.; Oh, C. H. Chem. Commun. 2005, 4429.
[22] Oh, C. H.; Lee, J. H.; Lee, S. J.; Kim, J. I.; Hong, C. S. Angew. Chem., Int. Ed. 2008, 47, 7505.
[23] Oh, C. H.; Lee, J. H.; Lee, S. M.; Yi, H. J.; Hong, C. S. Chem. Eur. J. 2009, 15, 71.
[24] Oh, C. H.; Lee, S. M.; Hong, C. S. Org. Lett. 2010, 12, 1308.
[25] Oh, C. H.; Tak, S. Y.; Lee, J. H.; PiaoBull, L. Bull. Korean Chem. Soc. 2011, 32, 2978.
[26] Kim, J. H.; Ray, D.; Hong, C. S.; Hana, J. W.; Oh, C. H. Chem. Commun. 2013, 49, 5690.
[27] Zheng, H.-J.; Zheng, J.-J.; Yu, B.-X.; Chen, Q.; Wang, X.-L.; He, Y.-P.; Yang, Z.; She, X.-G. J. Am. Chem. Soc. 2010, 132, 1788.
[28] Saito, K.; Sogou, H.; Suga, T.; Kusama, H.; Iwasawa, N. J. Am. Chem. Soc. 2011, 133, 689.
[29] (a) Nagata, T.; Nakagawa, M.; Nishida, A. J. Am. Chem. Soc. 2003, 125, 7484. (b) Young, I. S.; Williams, J. L.; Kerr, M. A. Org. Lett. 2005, 7, 953. (c) Young, I. S.; Kerr, M. A. J. Am. Chem. Soc. 2007, 129, 1465. (d) Deng, H.-B.; Yang, X.-B.; Tong, Z.-L.; Li, Z.; Zhai, H.-B. Org. Lett. 2008, 10, 1791. (e) Jakubec, P.; Cockfield, D. M.; Dixon, D. J. J. Am. Chem. Soc. 2009, 131, 16632. (f) Inagaki, F.; Kinebuchi, M.; Miyakoshi, N.; Mukai, C. Org. Lett. 2010, 12, 1800. (g) Cheng, B.; Wu, F.-F.; Yang, X.-B.; Zhou, Y.-D.; Wan, X.-L.; Zhai, H.-B. Chem. Eur. J. 2011, 17, 12569.
[30] (a) Skuballa, W.; Vorbrüggen, H. Angew. Chem., Int. Ed. 1981, 20, 1046. (b) Nickolson, R. C.; Vorbrüggen, H. Tetrahedron Lett. 1983, 24, 47.
[31] He, T.; Gao, P.; Zhao, S.-C.; Shi, Y.-D.; Liu, X.-Y.; Liang, Y.-M. Adv. Synth. Catal. 2013, 355, 365.
[32] Hsu, Y.-C.; Ting, C.-M.; Liu, R.-S. J. Am. Chem. Soc. 2009, 131, 2090.
[33] Radomkit, S.; Sarnpitak, P.; Tummatorn, J.; Batsomboon, P.; Ruchirawat, S.; Ploypradith, P. Tetrahedron 2011, 67, 3904.
[34] William, T.; Kelsey, J.; Jordan, G.; Neil, C. Adv. Org. Synth. 2013, 6, 59.
[35] (a) Conner, M. L.; Xu, Y.; Brown, M. K. J. Am. Chem. Soc. 2015, 137, 3482. (b) Jia, M.-Q.; Monari, M.; Yang, Q.-Q.; Bandini, M. Chem. Commun. 2015, 51, 2320. (c) Li, X.-X.; Zhu, L.-L.; Zhou, W.; Chen, Z. Org. Lett. 2012, 14, 1185.
[36] Ebisawa, M.; Kusama, H.; Iwasawa, N. Chem. Lett. 2012, 41, 786.
[37] Bigeault, J.; Giordano, L.; Riggi, I. D.; Gimbert, Y.; Buono, G. Org. Lett. 2007, 9, 3567.
[38] Achard, T.; Lepronier, A.; Gimbert, Y.; Clavier, H.; Giordano, L.; Tenaglia, A.; Buono, G. Angew. Chem. 2011, 123, 3614.
[39] Shafiee, A.; Ahond, A.; Bui, A. M.; Langlois, Y.; Riche, C.; Potier, P. Tetrahedron Lett. 1976, 17, 921.
[40] Andriantsiferana, M.; Besselievre, R.; Riche, C.; Husson, H.-P. Tetrahedron Lett. 1977, 18, 2587.
[41] (a) Carroll, A. R.; Hyde, E.; Smith, J.; Quinn, R. J.; Guymer, G.; Forster, P. I. J. Org. Chem. 2005, 70, 1096. (b) Taniguchi, T.; Martin, C. L.; Monde, K.; Nakanishi, K.; Berova, N.; Overman, L. E. J. Nat. Prod. 2009, 72, 430.
[42] Kusama, H.; Sogo, H.; Saito, K.; Suga, T.; Iwasawa, N. Synlett 2013, 24, 1364.
[43] Shu, D.-X.; Song, W.-Z.; Li, X.-X.; Tang, W.-P. Angew. Chem., Int. Ed. 2013, 52, 3237.
[44] Trillo, B.; López, F.; Gulías, M.; Castedo, L.; Mascareñas, J. L. Angew. Chem., Int. Ed. 2008, 47, 951.
[45] Tenaglia, A.; Gaillard, S. Angew. Chem., Int. Ed. 2008, 47, 2454.
[46] Okamoto, K.; Hayashi, T. Org. Lett. 2007, 9, 5067.
[47] Wang, X.; Xu, X.; Zavalij, P. Y.; Doyle, M. P. J. Am. Chem. Soc. 2011, 133, 16402.
[48] Yang, W.; Wang, T.; Yu, Y.; Shi, S.; Zhang, T.; Hashmi, A. S. K. Adv. Synth. Catal. 2013, 355, 1523.
[49] Blanco-Urgoiti, J.; Añorbe, L.; Pérez-Serrano, L.; Domínguez, G.; Pérez-Castells, J. Chem. Soc. Rev. 2004, 33, 32.
[50] Murakami, M.; Itami, K.; Ito, Y. J. Am. Chem. Soc. 1999, 121, 1430.
[51] Mazumder, S.; Crandell, D. W.; Lord, R. L.; Baik, M.-H. J. Am. Chem. Soc. 2014, 136, 9414.
/
| 〈 |
|
〉 |