

Chinese Journal of Organic Chemistry >
Michael Addition and Tandem Reaction of Aminothiphenols with α,β- Unsaturated Ketones with High Steric Hindrance Catalyzed by CeCl3·7H2O-NaI Supported on SiO2 Microsphere
Received date: 2015-04-30
Revised date: 2015-07-06
Online published: 2015-07-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 20972040).
Using SiO2 microsphere with size of 5 μm as support, CeCl3·7H2O-NaI/SiO2 supported catalyst was prepared and characterized by SEM, EDS and XDR techniques and its catalytic performance for Michael addition and tandem Michael addition/condensation reactions between aminothiophenols and α,β-unsaturated ketones with high steric hindrance were investigated. The experimental results showed that CeCl3·7H2O-NaI/SiO2 can catalyzed above reactions in moderate to good yields. When the catalyst was used fifth times, its morphology was largely unchanged, but its catalytic activity decreased for losing of small amounts of metal cerium in the process of catalyzing. It was also found that CeCl3·7H2O-NaI/SiO2 can catalyze both the Michael addition of aminothiophenols to α,β-unsaturated ketones and the condensation reaction between ketones and amines.
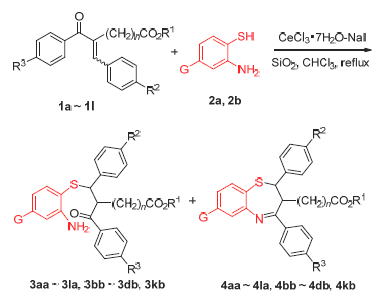
Key words: Michael addition; tandem reaction; CeCl3·; 7H2O-NaI; SiO2 microsphere; supported catalyst
Zhang Yiyang , Mu Boshuai , Li Yuan . Michael Addition and Tandem Reaction of Aminothiphenols with α,β- Unsaturated Ketones with High Steric Hindrance Catalyzed by CeCl3·7H2O-NaI Supported on SiO2 Microsphere[J]. Chinese Journal of Organic Chemistry, 2015 , 35(11) : 2347 -2357 . DOI: 10.6023/cjoc201504047
[1] En, D.; Zou, G. F.; Guo, Y.; Liao, W. W. J. Org. Chem. 2014, 79(10), 4456.
[2] Bartoli, G.; Bartolacci, M.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Torregiani, E. J. Org. Chem. 2005, 70(1), 169.
[3] Garg, S. K.; Kumar, R.; Chakraborti, A. K. Tetrahedron Lett. 2005, 46(10), 1721.
[4] Kawatsura, M.; Komatsu, Y.; Yamamoto, M.; Hayase, S.; Itoh, T. Tetrahedron 2008, 64(16), 3488.
[5] Keithellakpam, S.; Laitonjam, W. S. Chin. Chem. Lett. 2014, 25, 767.
[6] Li, Z.-F.; Hou, H.-L.; Ying, A.-G.; Xu, S. -L. Chin. J. Org. Chem. 2014, 34, 1074 (in Chinese)(李志峰, 侯海亮, 应安国, 许松林, 有机化学, 2014, 34, 1074.)
[7] Hou, X.-H.; Ma, Z.-W.; Wang, J.-L.; Liu, H.-M. Chin. J. Org. Chem. 2014, 34, 1509 (in Chinese).(侯学会, 马志伟, 王建玲, 刘宏民, 有机化学, 2014, 34, 1509.)
[8] Ma, A.; Ma, D. W. Org. Lett. 2010, 12(16), 3634.
[9] Hong, B. C.; Kotame, P.; Tsai, C. W.; Liao, J. H. Org. Lett. 2010, 12(4), 776.
[10] Enders, D.; Wang, C.; Bats, J. W. Synlett 2009, 1777.
[11] Wang, W.; Li, H.; Wang, J.; Zu, L. J. Am. Chem. Soc. 2006, 128, 10354.
[12] Tan, B.; Lu,Y.; Zeng, X.; Chua, P. J.; Zhong, G. Org. Lett. 2010, 12(12), 2682.
[13] Zheng, D.; Li, S.; Luo, Y.; Wu, J. Org. Lett. 2011, 13(24), 6402.
[14] Ye, L.; Sun, X.; Zhu, C.; Tang, Y. Org. Lett. 2006, 8(17), 3853.
[15] Yu, L.; Wu, L. L.; Xie, M. H.; Shi, L.; Huang, X. Chin. J. Struct. Chem. 2005, 24(12), 1408.
[16] Yu, L.; Chen, Z. H. Synth. Commun. 2005, 35, 1253.
[17] Dai, X. Y.; Wu, X. Y.; Fang, H. H.; Nie, L. L.; Chen, J.; Deng, H. M.; Cao, W. G.; Zhao, G. Tetrahedron 2011, 67(17), 3034.
[18] Wu, X. Y.; Fang, H. H.; Liu, Q.; Nie, L. L.; Chen, J.; Cao, W. G.; Zhao, G. Tetrahedron 2011, 67(38), 7251.
[19] Qiu, Z. -L.; Li, W. -H.; Zhu, H. -F.; Liu, Q.; Li, Y. Chem. J. Chin. Univ.2013, 34(3), 579 (in Chinese). (邱召来, 李文红, 朱海菲, 刘倩, 李媛, 高等学校化学学报, 2013, 34(3), 579.)
[20] Franzén, J.; Fisher, A. Angew. Chem., Int. Ed. 2009, 48, 787.
[21] Zhang, W.; Franzén, J. Adv. Synth. Catal. 2010, 352, 499.
[22] Wu, X. Y.; Zhang, Y.; Dai, X. Y.; Fang, H. H.; Chen, J.; Cao, W. G.; Zhao, G. Synthesis 2011, 3675.
[23] Liu, Q.; Li, W.-H.; Qiu, Z.-L.; Li, Y. Chem. J. Chin. Univ.2014, 35(3), 538 (in Chinese). (刘倩, 李文红, 邱召来, 李媛, 高等学校化学学报, 2014, 35(3), 538.)
[24] Gao, L.-Y. M.S. Dissertation, Hebei Normal University, Shijiazhuang, 2014 (in Chinese).(高丽叶, 硕士论文, 河北师范大学, 石家庄, 2014.)
/
| 〈 |
|
〉 |