

Chinese Journal of Organic Chemistry >
4,6-2,1,3-Benzothiadiazole: A New Block to Construct Polymer Optoelectrical Functional Materials and Multiple Performance Regulator
Received date: 2015-01-23
Revised date: 2015-02-13
Online published: 2015-07-24
Supported by
Project supported by the Science and Technology Research Key Project of Education Department of Henan Province (No. 14A150009), the Science and Technology Plan Project of Zhengzhou City (No. 141PPTGG398) and the Doctor Research Project of Zhengzhou University of Light Industry (No. 2013BSJJ017).
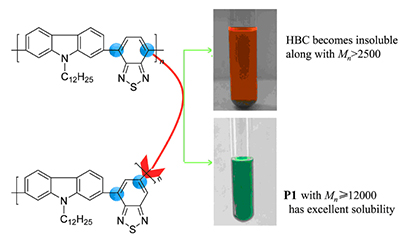
Poly(2,7-N-dodecyl-carbazole)-co-(4,6-2,1,3-benzothiadiazole) (P1) was synthesized for the first time with Suzuki condensation polymerization by introducing 4,6-2,1,3-benzothiadiazole unit. The UV, PL spectra and cyclic voltammetry (CV) curve of P1 were tested. Compared with its 4,7-linked polymer HBC, the higher molecular weight Mn=12000 of P1 was obtained. The UV and PL spectrum shown that the maximum absorption wavelength and photoluminescence of P1 in toluene solution had blue shift about 78 and 43 nm, respectively. The optical bandgap and electrical bandgap increased about 0.35 and 0.40 eV, respectively while the shape of CV curves were similar. It suggested that the solubility of benzothiadiazole based polymer materials was improved effectively and the optoelectrical properties were tunable efficiently by introduction of 4,6-benzothiadiazole unit. It would supply a new method to realize multiple performance tunable of benzothiadaizole based polymer materials.
Fang Shaoming , Su Jigong , Wang Guohao , Xue Likun , Zhang Yonghui , Du Junping . 4,6-2,1,3-Benzothiadiazole: A New Block to Construct Polymer Optoelectrical Functional Materials and Multiple Performance Regulator[J]. Chinese Journal of Organic Chemistry, 2015 , 35(7) : 1565 -1569 . DOI: 10.6023/cjoc201501033
[1] Quites, F. J.; Faria, G. C.; Germino, J. C.; Atvars, T. D. Z. J. Phys. Chem. A 2014, 118, 10380.
[2] Zhang, C.; Sun, Y.; Dai, B.; Zhang, X.-Q.; Yang, H.; Lin, B.-P.; Guo, L.-X. Chin. J. Org. Chem. 2014, 34, 1701 (in Chinese). (张超, 孙莹, 戴斌, 张雪勤, 杨洪, 林保平, 郭玲香, 有机化学, 2014, 34, 1701.)
[3] Peng, J.; Xu, X.; Tian, Y.; Wang, J.; Tang, F.; Li, L. Appl. Phys. Lett. 2014, 105, 173301
[4] Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Nat. Commun. 2014, 5, 5293.
[5] Dang, D.; Zhou, P.; Zhong, J.; Fan, J.; Wang, Z.; Wang, Y.; Pei, Y.; Bao, X.; Yang, R.; Hu, W.; Zhu, W. Polymer 2014, 55, 6708.
[6] Dabeux, F.; Caille, J.-R.; Fenoll, M.; Bolsee, J.-C. EP 2808373, 2014 [Chem. Abstr. 2014, 162, 45318].
[7] Scharber, M. C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A. J.; Brabec, C. J. Adv. Mater. 2006, 18, 789.
[8] Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletête, M.; Durocher, G.; Tao, Y.; Leclerc, M. J. Am. Chem. Soc. 2008, 130, 732.
[9] (a) Bundgaard, E.; Krebs, F. Sol. Energy Mater. Sol. Cells 2007, 91, 954.(b) Sun, M.; Zhou, M.; Liang, L.; Wang, W.; Wang, W.; Ling, Q. Chin. J. Org. Chem. 2013, 33, 2504 (in Chinese).(孙敏敏, 周铭露, 梁露英, 王维, 王文, 凌启淡, 有机化学, 2013, 33, 2504.)(c) Liu, Z.; Xu, F.; Yan, D. Acta Chim. Sinica 2014, 72, 171 (in Chinese).(刘震, 徐丰, 严大东, 化学学报, 2014, 72, 171.)(d) Xia, X.; Lei, T.; Pei, J.; Liu, C. Chin. J. Org. Chem. 2014, 34, 1905 (in Chinese).(夏昕, 雷霆, 裴坚, 刘晨江, 有机化学, 2014, 34, 1905.)(e) He, P.; Li, Z.; Hou, Q.; Wang, Y. Chin. J. Org. Chem. 2013, 33, 288 (in Chinese).(和平, 李在房, 侯秋飞, 王艳玲, 有机化学, 2013, 33, 288.)
[10] Kim, J.; Han, A.-R.; Hong, J.; Kim, G.; Lee, J.; Shin, T. J.; Oh, J. H.; Yang, C. Chem. Mater. 2014, 26, 4933.
[11] Yu, L.; Liu, J.; Hu, S.; He, R.; Yang, W.; Wu, H.; Peng, J.; Xia, R.; Bradley, D. D. C. Adv. Funct. Mater. 2013, 23, 4366.
[12] Kowalski, S.; Allard, S.; Scherf, U. ACS. Macro Lett. 2012, 1, 465.
[13] Yoon, K.-J.; Park, J. S.; Lee, S.-J.; Song, M.; Shin, I. A.; Lee, J. W.; Gal, Y.-S.; Jin, S.-H. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 6762.
[14] Park, J. S.; Jin, S.-H.; Gal, Y.-S.; Lee, J. H.; Lee, J. W. Mol. Cryst. Liq. Cryst. 2012, 567, 102.
[15] Mitchell, W.; Tiemey, S.; Wang, C.; Blouin, N. WO 2011098113, 2013 [Chem. Abstr. 2013, 155, 301427].
[16] Larsen-olsen, T. T.; Bundgaard, E.; Sylvester-Hvid, K. O.; Krebs, F. C. Org. Electron. 2011, 12, 364.
[17] Zhou, M.; Wang, P.; Zhang, Z.; Liang, L. WO 2012088698, 2012 [Chem. Abstr. 2012, 157, 134230].
[18] Lu, Z.; Li, C.-H.; Du, C.; Gong, X.; Bo, Z.-S. Chin. J. Polym. Sci. 2013, 31, 901.
[19] Qin, R.; Li, W.; Li, C.; Du, C.; Veit, C.; Schleiermacher, H.-F.; Andersson, M.; Bo, Z.; Liu, Z.; Inganas, O.; J. Am. Chem. Soc. 2009, 131, 14612.
[20] Li, Y.; Zou, J.; Yip, H.-L.; Li, C.-Z.; Zhang, Y.; Chueh, C.-C.; Intermann, J.; Xu, Y.; Liang, P.-W.; Chen, Y.; Jen, A. K.-Y. Macromolecules 2013, 46, 5497.
[21] Jwo, P.-C.; Lai, Y.-Y.; Tsai, C.-E.; Lai, Y.-Y.; Liang, W.-W.; Hsu, C.-S.; Cheng, Y.-J. Macromolecules 2014, 47, 7386.
[22] Du, J.; Xu, E.; Zhong, H.; Yu, F.; Liu, C.; Wu, H.; Zeng, D.; Ren, S.; Sun, J.; Liu, Y.; Cao, A.; Fang, Q. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 1376.
[23] Pilgram, K.; Skiles, R. D. J. Heterocycl. Chem. 1974, 11, 777.
[24] Pesin, V. G.; Khaletskii, A. M. Zh. Obshch. Khim. 1957, 27, 2599.
[25] Manley, P. W.; Acemoglu, M.; Marterer, W.; Pachinger, W. Org. Process Res. Dev. 2003, 7, 436.
[26] Huang, Y.; Wen, W.; Mukherjee, S.; Ade, H.; Kramer, E. J.; Bazan, G. C. Adv. Mater. 2014, 26, 4168.
[27] Li, W.; Yang, L.; Tumbleston, J. R.; Yan, L.; Ade, H.; You, W. Adv. Mater. 2014, 26, 4456. Kuwabara, J.; Yasuda, T.; Choi, S. J.; Lu, W.; Yamazaki, K.; Kagaya, S.; Han, L.; Kanbara, T. Adv. Funct. Mater. 2014, 24, 3226.
/
| 〈 |
|
〉 |