

Chinese Journal of Organic Chemistry >
Synthesis and Property of Unsymmetric Bent-core Liquid Crystals Based on 2,5-Disubstitueted-1,3,4-oxadiazole
Received date: 2015-04-18
Revised date: 2015-07-05
Online published: 2015-08-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 11074054, 11374067).
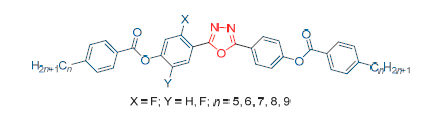
Two novel series of bent-core liquid crystals (9a~9e and 10a~10e) were prepared with a central bent core based on 2,5-disubstitueted-1,3,4-oxadiazole, ester group bridge (coo), alkyl tails with different carbon numbers (n=5~9), and one side wing substituted by fluorine atom. The structures of the target compounds were confirmed by IR, 1H NMR, 13C NMR, MS, and their transition temperatures and phase textures were investigated by using differential scanning calorimetric (DSC) and polarizing optical microscopy (POM). The result suggested that all target compounds had right chemical structure. All compounds 9a~9e and 10a~10e exhibited nematic phase and existed wide temperature range of nematic phase. The widest range of nematic phase was 78 ℃. The temperature range of nematic phase of compounds 9a~9e with lateral monofluoro-substituted benzene was more narrow than compounds 10a~10e with lateral difluoro-substituted benzene. Moreover, compounds 9a~9e showed odd-even effect.
Key words: oxadiazole; unsymmetric bent-core liquid crystal; nematic phase; synthesis
Mao Zhipeng , You Hongjun , Wang Kun , Zhang Zhiyong , Guan Jintao , Dai Zhiqun , Xiang Ying . Synthesis and Property of Unsymmetric Bent-core Liquid Crystals Based on 2,5-Disubstitueted-1,3,4-oxadiazole[J]. Chinese Journal of Organic Chemistry, 2015 , 35(12) : 2575 -2582 . DOI: 10.6023/cjoc201504026
[1] Nori, T.; Sekine, T.; Watanabe, J. J. Mater. Chem. 1996, 6, 1231.
[2] Link, D. R.; Natale, G.; Shao, R. F. Science 1997, 278, 1924.
[3] Madsen, L. A.; Dingemans, T. J.; Nakata, M. Phys. Rev. Lett. 2004, 92, 145506.
[4] Taushanoff, S.; Le, K. V.; Williams, J. J. Mater. Chem. 2010, 20, 5893.
[5] Prasad, V.; Kang, S. W.; Suresh, K. A. J. Am. Chem. Soc. 2005, 127, 17224.
[6] Gleeson, H. F.; Kaur, S.; Görtz, V. ChemPhysChem 2014, 15, 1251.
[7] Du, Q.; You, H.-J.; Wang, X.-Y.; Zhang, Z.-Y.; Dai, Z.-Q. Liq. Cryst. Disp. 2011, 26, 719 (in Chinese). (杜琼, 游红军, 汪晓燕, 张智勇, 戴志群, 液晶与显示, 2011, 26, 719.)
[8] Görtz, V.; Southern, C.; Roberts, N. W.; Gleeson, H. F.; Goodby, J. W. Soft Matter 2009, 5, 463.
[9] Cooper, L. L.; Samulski, E. T.; Scharrer, E. Mol. Cryst. Liq. Cryst. 2009, 511, 203.
[10] Speetjens, F.; Lindborg, J.; Tauscher, T. J. Mater. Chem. 2012, 22, 22558.
[11] Wang, Y.-L.; Du, Q.; Wang, G.-H.; Zhang, Z.-Y. Chin. J. Org. Chem. 2013, 33, 2010 (in Chinese). (王勇丽, 杜琼, 王国华, 张智勇, 有机化学, 2013, 33, 2010.)
[12] Maksimenko, S. I.; Novikove, N. S.; Yarkove, M. Y. Russ. J. Org. Chem. 2007, 43, 1773.
[13] Yoshida, M.; Otaka, H.; Doi, T. Eur. J. Org. Chem. 2014, 27, 6010.
[14] Huang, H.-M.; Yu, H,-T.; Chen, P.-L. Chin. J. Org. Chem. 2004, 24, 502 (in Chinese). (黄华鸣, 于海涛, 陈培丽, 有机化学, 2004, 24, 502.)
[15] Neubert, M. E. Mol. Cryst. Liq. Cryst. 1979, 53, 101.
[16] Zhu, D.-X.; Lu, H.-F.; Zhang, J. CN 101643456, 2009 [Chem. Abstr. 2009, 152, 287388].
[17] You, H.-J. Bachelor Thesis, Wuhan Polytechnic University, Wuhan, 2009 (in Chinese). (游红军, 学士论文, 武汉工业学院, 武汉, 2009.)
[18] Huang, P.; Hu, P.-L.; Shen, D. Liq. Cryst. Disp. 2010, 25, 626 (in Chinese). (黄佩, 胡萍兰, 沈冬, 液晶与显示, 2010, 25, 626.)
/
| 〈 |
|
〉 |