

Chinese Journal of Organic Chemistry >
A New Method for the Synthesis of Alkyl-Substituted 1,2,3-Triazole Compounds
Received date: 2015-05-27
Revised date: 2015-08-09
Online published: 2015-08-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172059, 21372066, 21402041), the Research Fund for the Doctoral Program of Higher Education (No. 20124104110006), and the Creative Experimental Project of National Undergraduate Students (Nos. 201310476035, 201410476027).
1,2,3-Trizaole derivatives have been widely used in the fields of medicine pesticide and materials. Thus, much attention has been paid to the synthesis of various 1,2,3-trizaole derivatives. However, the transformation of function group at N(1) position of 1,2,3-triazole derivatives was less studied. In this paper, in the presence of base and using copper salt as the catalyst, the sulfonyl group of 1,2,3-triazole derivatives could be transformed to other function groups in a "one-pot two-steps" manner, including benzyl, substituted benzyl, halide substituted alkyl or alkyl groups. This method exhibited mild reaction conditions and simple operation. Meanwhile, based on the captured reaction intermediate, the reaction mechanism was also proposed.
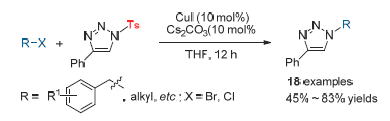
Xie Mingsheng , Tang Haoxin , Zhang Yiming , Guo Zhen , Guo Haiming , Qu Guirong . A New Method for the Synthesis of Alkyl-Substituted 1,2,3-Triazole Compounds[J]. Chinese Journal of Organic Chemistry, 2015 , 35(12) : 2589 -2594 . DOI: 10.6023/cjoc201505040
[1] (a) Katritzky, A. R.; Singh, S. K. J. Org. Chem. 2002, 67, 9077. (b) Malow, M.; Wehrstedt, K. D.; Neuenfeld, S. Tetrahedron Lett. 2007, 48, 1233. (c) Jiang, Y. B.; Kuang, C. X. Prog. Chem. 2012, 24, 1983. (d) Chen, Z.; Yan, Q.; Yi, H.; Liu, Z.; Lei, A.; Zhang, Y. Chem. Eur. J. 2014, 20, 13692. (e) Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Org. Lett. 2014, 16, 5728. (f) Velázquez, H. D.; García, Y. R.; Vandichel, M.; Madder, A.; Verpoort, F. Org. Biomol. Chem. 2014, 12, 9350. (g) Huo, J.; Wei, X.; Mo, G.; Peng, P.; Zhong, M.; Chen, R.; Wang, Z. Chin. J. Org. Chem. 2014, 34, 92 (in Chinese). (霍景沛, 韦新平, 莫广珍, 彭湃, 钟铭丽, 陈任宏, 汪朝阳 有机化学, 2014, 34, 92.) (h) Jiang, Y.; Han, C.; Liang, X.; Yang, P.; Wang, H. Chin. J. Org. Chem. 2013, 33, 1884 (in Chinese). (江玉波, 韩春美, 梁雪秋, 杨朋, 王红, 有机化学, 2013, 33, 1884.)
[2] (a) Ju, W.; Chen, G.; Hu, Y.; Zhang M. Chin. J. Pharm. 2010, 41, 247. (b) Soltis, M. J.; Yeh, H. J.; Cole, K. A.; Whittaker, N.; Wersto, R. P.; Kohn, E. C. Drug Mettab. Dispos. 1996, 24, 799. (c) Whiting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S. M.; Lindstrom, W.; Olson, A. J.; Kolb, H. C.; Finn, M. G.; Sharpless, K. B.; Elder J. H.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 1435. (d) Micetich, R. G.; Maiti, S. N.; Spevak, P.; Hall, T. W.; Yamabe, S.; Ishida, N.; Tanaka, M.; Yamazaki, T.; Nakai, A.; Ogawa, K. J. Med. Chem. 1987, 30, 1469.
[3] (a) Michael, A. J. Prakt. Chem. 1893, 48, 94. (b) Huisgen, R. 1,3-Dipolar Cycloaddition Chemistry, Wiley, New York, 1984, p. 1. (c) Amblard, F.; Cho, J. H.; Schinazi, R. F. Chem. Rev. 2009, 109, 4207.
[4] (a) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. (b) Wang, Q.; Chan, T. R.; Hilgraf, R.; Fokin, V. V.; Sharpless, K. B.; Finn, M. G. J. Am. Chem. Soc. 2003, 125, 3192.
[5] (a) Chan, T. R.; Hilgraf, R.; Sharpless, K. B.; Fokin, V. V. Org. Lett. 2004, 6, 2853. (b) Appukkuttan, P.; Dehaen, W.; Fokin, V. V.; Eycken, E. V. Org. Lett. 2004, 6, 4223.
[6] (a) Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. (b) Zhao, Y.-B.; Yan, Z.-Y.; Liang, Y.-M. Tetrahedron Lett. 2006, 47, 1545. (c) Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923. (d) Li, P.; Wang, L.; Zhang, Y. Tetrahedron 2008, 64, 10825. (e) Kwok, S. W.; Fotsing, J. R.; Fraser, R. J.; Rodionov, V. O.; Fokin, V. V. Org. Lett. 2010, 12, 4217. (f) Belkheira, M.; Abed, D. E.; Pons, J.; Bressy, C. Chem.-Eur. J. 2011, 17, 12917.
[7] (a) Raushel, J.; Fokin, V. V. Org. Lett. 2010, 12, 4952. (b) Yamauchi, M.; Miura, T.; Murakami, M. Heterocycles 2010, 80, 177.
/
| 〈 |
|
〉 |