

Chinese Journal of Organic Chemistry >
Synthesis and Cytotoxic Activity of Imatinib Derivatives
Received date: 2015-06-25
Revised date: 2015-07-27
Online published: 2015-08-26
Supported by
Project supported by the Foundation of Heilongjiang Educational Committee (No. 12521417).
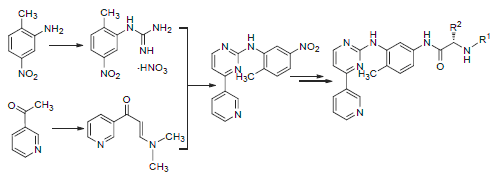
Fourteen derivatives of imatinib have been prepared by condensation of (L)-N-acylation amino acid with N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidine amine (6), which was prepared from 3-acetyl-pyrimidin and 2-methyl-5-nitroaniline through the reactions of addition, condensation, cyclization and reduction, respectively. The structures of all target compounds were characterized by IR, 1H NMR, 13C NMR and HRMS techniques. They were evaluated for cytotoxic activity against human Leukemia cells (K562), human non-small-cell-lung cancer cells lines (A549) and human hepatoma cell lines (HepG-2) by methyl thiazolyl tetrazolium (MTT) method. The results showed the cytotoxic activities of compounds 7d,7i,7j,7k,7l, 7n against human Leukemia cells (K562) and human non-small-cell-lung cancer cell lines (A549), compounds 7a, 7d,7e against human hepatoma cell lines (HepG-2) were comparable to those of imatinib.
Chen Shijie , He Long , Wang Xuewei , Gong Xianfeng , Zhang Hua . Synthesis and Cytotoxic Activity of Imatinib Derivatives[J]. Chinese Journal of Organic Chemistry, 2015 , 35(11) : 2377 -2382 . DOI: 10.6023/cjoc201506030
[1] Guo, X.-N.; Ding, J. Chin. J. Med. Chem. 2003, 34, 183 (in Chinese).(郭晓宁, 丁健, 中国新药杂志, 2003, 34, 183.)
[2] Wang, S. H. J. Leuk. Lymphoma 2002, 11, 29 (in Chinese).(张穗慧, 白血病·淋巴瘤, 2002, 11, 29.)
[3] Guilhot, F.; Chastang, C.; Michallet, M.; Guercl, A.; Harousseallu, J. L.; Maloisel, F.; Bouabdallah, R.; Gugotat, D.; Cheron, N.; Nicolini, F.; Abgraii, J. F.; Tanzer, J. N. Engl. J. Med. 1997, 337, 223..
[4] Nowell, P. C.; Hungerford, D. A. Science 1960, 132, 1497.
[5] Chronic Myeloid Leukemia Trialists' Collaborative Group J. Natl. Cancer Inst. 1997, 89, 1616.
[6] O'Brien, S. G.; Guilhot, F.; Larson, R. A. N. Engl. J. Med. 2003, 348, 994.
[7] Gambacorti Passerini, C. B.; Gunby, R. H.; Piazza, R. J. Lancet Oncol. 2003, 4, 75.
[8] Mahon, F. X.; Faberes, C.; Pueyo, S. Blood 1998, 92, 4059.
[9] Zheng, Y.-G.; Chen, D.-J.; Zhu, B.-Q. Chin. J. Med. Chem. 2009, 18, 189 (in Chinese). (郑玉果, 陈代杰, 朱宝泉, 中国新药杂志, 2009, 18, 189.)
[10] Sawyers, C. L.; Hohhasus, A.; Feldman, E.; Goldman, J. M.; Miller, C. B.; Ottmann, O. G.; Schiffer, C. A.; Talpaz, M.; Guilhot, F.; Deininger, M. W. N.; Fischer, T.; OBrien, S. G.; Stone, R. M. Blood 2002, 99, 3530.
[11] Kil, K. E.; Ding, Y. S.; Lin, K. S. Nucl. Med. Biol. 2007, 34, 153.
[12] Kompella, A. K.; Adibnatla, K. S.; Bhujanga, R. WO 2006027795, 2006 [Chem. Abstr. 2006, 144, 312102].
[13] Buerger, H. M.; Caravatti, G.; Zimmerman, J. WO 2002022597, 2002 [Chem. Abstr. 2002, 136, 2476057].
[14] Jur, G. Z.; Wallba, C. H. US 5521184, 1996 [Chem. Abstr. 1996, 125, 114681].
[15] Li, M.-D.; Li, D.; Ji, M. Chin. Pharm. J. 2008, 43, 228 (in Chinese).(李铭东, 李东, 吉民, 中国药学杂志, 2008, 43, 228.)
/
| 〈 |
|
〉 |