

Chinese Journal of Organic Chemistry >
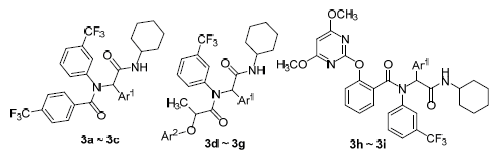
Synthesis of N-Cyclohexyl 2-{N-(3-Trifluoromethylphenyl)-[3-(trifluo-romethyl)benzoyl, 2-(substitutedphenoxy)propionyl, 2-(4,6-dime-thoxypyrimidinyl-2-oxy)benzoyl]-amino}-substituted-phenyl-acetamides via Ugi Reaction and Their HerbicidalActivity Evaluation
Received date: 2015-07-03
Revised date: 2015-07-22
Online published: 2015-08-31
Supported by
Project supported by the National Natural Science Foundation of China (No. 21342004) and the Natural Science Foundation of Hubei Province (No. 2015CKB741).
In order to find novel high active herbicidal lead compound, a series of N-cyclohexyl 2-{N-(3-trifluoromethyl-phenyl)-[3-(trifluoromethyl)benzoyl, 2-(substitutedphenoxy)propionyl, 2-(4,6-dimethoxypyrimidinyl-2-oxy)benzoyl]-amino}-substitutedphenylacetamides 3a~3i were designed and synthesized by introducing 3-trifluoromethylbenzoyl, 2-aryloxy-pro-pionyl or 2-(4,6-dimethoxypyrimidin-2-yloxy)benzoyl and 3-trifluoromethyl phenyl moieties into the molecular skeleton via the Ugi reaction. Their structures were characterized by IR, 1H NMR, EI-MS, and elemental analyses. The preliminary bioassay (in vitro) indicated that some of compounds 3 displayed moderate to good herbicidal activity against Brassica campestris L at the concentration of 100 mg/L. The glasshouse bioassay (in vivo) showed that some of compounds 3 exhibited good herbicidal activities against B. campestris, A. retroflexus, E. crusgalli and D. sanguinalis in pre-emergence treatment at a dose of 1500 g/ha.
Hu Hanning , Li Anling , Zhang Hanyun , Shi Deqing . Synthesis of N-Cyclohexyl 2-{N-(3-Trifluoromethylphenyl)-[3-(trifluo-romethyl)benzoyl, 2-(substitutedphenoxy)propionyl, 2-(4,6-dime-thoxypyrimidinyl-2-oxy)benzoyl]-amino}-substituted-phenyl-acetamides via Ugi Reaction and Their HerbicidalActivity Evaluation[J]. Chinese Journal of Organic Chemistry, 2015 , 35(10) : 2162 -2167 . DOI: 10.6023/cjoc201507003
[1] Shimizu, T. J. Pestic. Sci. 1997, 22, 254.
[2] Tamaru, M.; Inoue, J.; Hanai, R. J. Agric. Food Chem. 1997, 45, 2777.
[3] Tamaru, M.; Takehi, T.; Masuyama, N. Pestic. Sci. 1996, 47, 327.
[4] Lu, L.; Chen, J.; Wu, J.; Ling, W.; Mao, L. S.; Li, M. Z.; Cai, X.; Peng, W. L.; Wu, Y.; Wu, S. G.; Wang, H. J.; Wang, G. C.; Cui, H.; Han, S. D.; Qiu, W. L.; Wang, Y. H. WO 2002034724, 2002 [ Chem. Abstr. 2002, 136, 355244].
[5] Wu, J.; Cheng, J.; Lu, L. J. Agric. Food Chem. 2006, 54, 5954.
[6] Wang, M. Q.; Ye, F. New Pestic. 2005, 39, 20 (in Chinese).(王铭琦, 叶非, 新农药, 2005, 39, 20.)
[7] Zhang, Y. J.; Li, J. D.; Zhang, D. S.; Sun, H. T.; Wang, W. X.; Ma, Q. X.; Pan, T. X.; Li, J. M. J. Henan Agric. Sci. 2001, 30(11), 13 (in Chinese).(张玉聚, 李继德, 张德胜, 孙化田, 王文夕, 马奇祥, 潘同霞, 李菊梅, 河南农业科学, 2001, 30(11), 13.)
[8] Sandmann, G.; Böger, P. In Phytoene Desaturase as a Target for Bleaching Herbicides: Herbicide Activity, Toxicology, Biochemistry, and Molecular Biology, Eds.: Roe, R. M.; Burton, J. D.; Kuhr, R. J., IOS Press, Amsterdam, 1997, pp. 1~10.
[9] Sandmann, G. In Herbicide Classes in Development: Mode of Action, Targets, Genetic Engineering, Chemistry, Eds.: Böger, P.; Wakabayashi, K.; Hirai, K., Springer, Berlin, 2002, pp. 43~55.
[10] Mitchell, G. In Synthesis and Chemistry of Agrochemicals IV, Eds.: Baker, D. A.; Fenyes, J. G.; Moberg, W. K.; Cross, B., ACS Symposium Series, American Chemical Society, Washington, DC, 1995, Vol. 584, pp. 161~170.
[11] Sandmann, G.; Kowalczyk-Schroder, S.; Taylor, H. M. Pestic. Biochem. Physiol. 1992, 42, 1.
[12] Sandmann, G.; Kunert, K. J.; Böger P. Pestic. Biochem. Physiol. 1981, 15, 28.
[13] Qian, X.; Lee, P. W.; Cao, S. J. Agric. Food Chem. 2010, 58, 2613.
[14] Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
[15] Marcaccini, S.; Torroba, T. Nat. Protoc. 2007, 3, 632.
[16] Ugi, I.; Werner, B.; Dömling, A. Molecules 2003, 1, 53.
[17] Zuo, X.; Mi, N.; Fan, Z.; Zheng, Q.; Zhang, H.; Wang, H.; Yang, Z. J. Agric. Food Chem. 2010, 58, 2755.
[18] He, H. W.; Wang, J.; Liu, Z. J.; Wan, S. Q.; Lu, A. H. Chin. J. Appl. Chem. 1994, 11(4), 21 (in Chinese).(贺红武, 汪军, 刘钊杰, 万树青, 陆爱红, 应用化学, 1994, 11(4), 21.)
[19] Li, Y. X. Fine Chem. Intermed. 2008, 38(4), 21 (in Chinese).(李元祥, 精细化工中间体, 2008, 38(4), 21.)
[20] Ugi, I.; Meyr, R.; Lipinski, M.; Bodesheim, F.; Rosendahl, F. Org. Synth. 1961, 41, 13.
[21] Chen, X. B.; Shi, D. Q.; Zhu, X. F. Chin. J. Chem. 2007, 25, 1854.
[22] Liu, Y. X.; Wei, D. G.; Zhu, Y. R.; Liu, S. H.; Zhang, Y. L.; Zhao, Q. Q.; Cai, B. L.; Li, Y. H.; Song, H. B.; Liu, Y.; Wang, Y.; Huang, R. Q.; Wang, Q. M. J. Agric. Food Chem. 2008, 56, 204.
/
| 〈 |
|
〉 |