

Chinese Journal of Organic Chemistry >
Synthesis and Characterization of the Coumarin-Binuclear Zinc Phthalocyanine Formed by Droxynaphthyl Bridge
Received date: 2015-08-11
Revised date: 2015-09-02
Online published: 2015-09-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 51272239), the National Natural Science Foundation of Shanxi Province (No. 2015011011) and the Shanxi Scholarship Council of China (No. 2015-078).
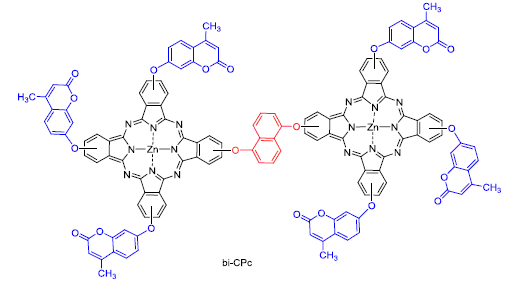
9(10),16(17),23(24)-Tris[4-methyl-coumarin-7-yloxy]-2(3)-(1,5-naphthalene) binuclear zinc phthalocyanine (bi-CPc) based 1,5-dihydroxynaphthalene, 4-nitro-phthalonitrile, resorcinol, ethyl acetoacetate and zinc acetate on raw material was synthesized. The structures and properties of precursors and bi-CPc were characterized by 1H NMR, FT-IR, UV-Vis, elemental analysis and thermogravimetric analysis. Results showed that strong π-π* transition was demonstrated by UV-Vis absorption spectrum of bi-CPc. At the same time, it existed in a form of monomer in solution of N,N-dimethylformamide (DMF) and possessed excellent thermal stability. Redox behavior of bi-CPc was researched by cyclic voltammetry (CV), and energy level of LUMO and HOMO of bi-CPc matched with energy level of nano-TiO2 after calculating. Through further structural modification, it is expected to use as photosensitive dye with excellent performance for solar cells.
Key words: phthalocyanine; binuclear; coumarin; dye-sensitized solar cells
Cao Jie , Liao Chaoqiang , Yang Ling , Zhang Xuejun . Synthesis and Characterization of the Coumarin-Binuclear Zinc Phthalocyanine Formed by Droxynaphthyl Bridge[J]. Chinese Journal of Organic Chemistry, 2016 , 36(1) : 213 -217 . DOI: 10.6023/cjoc201508013
[1] O'Regan, B.; Grätzel, M. Nature 1991, 353, 737
[2] Grätzel, M. J. Photochem. Photobiol. A 2004, 164, 34
[3] He, J.-J.; Chen, Y.-S.; Wang, T.-T.; Zeng, H.-P. Org. Chem. 2012, 32, 472 (in Chinese).(何俊杰, 陈舒欣, 王婷婷, 曾和平, 有机化学, 2012, 32, 472.)
[4] Hou, Y.-J.; Xie, P.-H.; Zhang, B.-W.; Cao, Y.; Xiao, X.-R.; Wang, W.-B. Inorg. Chem. 1999, 38, 6320.
[5] Horiuchi, T.; Miura, H.; Uchida, S. J. Photochem. Photobiol. A: Chem. 2004, 164, 29.
[6] Hara, K.; Kurashige, M.; Ito, S.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Chem. Commun. 2003, 252.
[7] Karthikeyan, C. S.; Peter, K.; Wietasch, H.; Thelakkat, M.; Energy, M. Sol. Cells 2007, 91, 432.
[8] Hara, K.; Sayama, K.; Ohga, Y.; Shinpo; Suga, S.; Arakawa, H. Chem. Commun. 2001, 569.
[9] Kitamura, T.; Ikeda, M.; Shigaki, K.; Inoue, T.; Andenson, N. A.; Ai, X.; Lian, T. Q.; Yanagida, S. Chem. Mater. 2004, 16, 1806.
[10] Hwang, S.; Lee, J. H.; Park, C.; Lee, H.; Kim, C.; Lee, M. H.; Lee, W.; Paka, J.; Kim, K.; Park, N. G.; Kim, C. Chem. Commun. 2007, 4887.
[11] Hara, K.; Miyamoto, K.; Abe, Y.; Yanagida, M. J. Phys. Chem. B 2005, 109, 23776.
[12] Wang, Y.-Q.; Lu, X.; Wei, X.-D.; Wu, W.-J.; Xie, Y.-S. New J. Chem.2014, 38: 3227.
[13] Mathew, S.; Yella, A; Gao, P.; Humphry-Baker, R. Nat. Chem. 2014, 6, 242.
[14] Takuro, L.; Hirotaka, N.; Naruhiko, M.; Matthew, J. G.; Shogo, M.; Mutsumi, K. Chem. Commun. 2014, 50, 1941.
[15] Mao, L.-J.; Tan, Q.-L.; Xing, G.-Q.; Han, M.-L.; Zhang, X.-J. Chin. J. Org. Chem. 2012, 32, 2315 (in Chinese).(毛利军, 谭青龙, 新冠琼, 韩明亮, 张学俊, 有机化学, 2012, 32, 2315.)
[16] Kc, C. C.; Stranius, K.; Dsouza, P. J. Chem. Phys. 2013, 117, 763.
[17] Camur, M.; Ahsen, V.; Durmus, M. Photochem. Photobiol. A: Chem. 2011, 219, 217.
[18] Han, L.; Zhou, X.; Ye, Q.; LI, J.-Y.; Gao, J.-R. Chin. J. Org. Chem.2013, 33, 1000 (in Chinese).(韩亮, 周雪, 叶青, 李郁绵, 高建荣, 有机化学, 2013, 33, 1000.)
[19] Peng, Y.-R.; Huang, F.-H. Strait Pharm. J. 2003, 15, 53 (in Chinese). (彭亦如, 黄风华, 海峡药学, 2003, 15, 53.)
[20] Han, M.-L. M.S. Thesis, North University of China, Taiyuan, 2015 (in Chinese).(韩明亮, 硕士论文, 中北大学, 太原, 2015.)
[21] Leeuw, D. M.; Simenon, M. M. J.; Brown, A. R. Synth. Met. 1997, 87, 53.
/
| 〈 |
|
〉 |