

Chinese Journal of Organic Chemistry >
Advance on Applications of Microwave Technique in Palladium- Catalyzed Suzuki-Miyaura Cross-Coupling Reaction
Received date: 2015-07-12
Revised date: 2015-08-24
Online published: 2015-09-06
Supported by
Project supported by the Science and Technology Department of Sichuan Province (No. 2015NZ0033) and the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities (No. 12NZYTH03).
Compared with conventional heating, microwave heating is one of the most useful tools in organic synthesis because of its obviously advantages of fast heating, thermal efficiency, saving energy, clean, and easy operation. Palladium catalyzed Suzuki-Miyaura cross-coupling reaction could tolerate a broad range of functional groups with high stereoselectivity to provide a mild method in preparation of kinds of substituted biaryls. In this paper, the recent research results about the microwave technique applied in Suzuki-Miyaura cross-coupling reaction are reviewed, involving various reaction systems. The applications of the methodology on the synthesis of natural products and bioactive molecules have also been introduced.
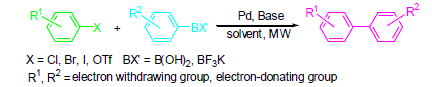
Li Qinghan , Ding Yong , Zhang Gang , Zhang Zhen , Mo Song . Advance on Applications of Microwave Technique in Palladium- Catalyzed Suzuki-Miyaura Cross-Coupling Reaction[J]. Chinese Journal of Organic Chemistry, 2016 , 36(1) : 83 -104 . DOI: 10.6023/cjoc201507008
[1] (a) Suzuki, A. J. Organomet. Chem. 1999, 576, 147. (b) Miyaura, N. Top. Curr. Chem. 2002, 219, 11.(c) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. (d) Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. (e) Kotha, S.; Lahiri, K.; Kashinath, D. Tetrahedron 2002, 58, 9633. (f) Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 2002, 41, 4176. (g) Miyaura, N. Top. Curr. Chem. 2002, 219, 11.(h) Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419. (i) Christmann, U.; Vilar, R. Angew. Chem., Int. Ed. 2005, 44, 366. (j) Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2008, 64, 3047.(k) de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions, Vol. 2, Wiley-VCH, Weinheim, 2004.(l) Ackermann, L. Modern Arylation Methods, Wiley-VCH, Weinheim, 2009.(m) Shen, X.; Jones, G. O.; Watson, D. A.; Bhayana, B.; Buchwald, S. L. J. Am. Chem. Soc. 2010, 132, 11278.(n) Lundin, P. M.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 11027.(o) Li, W.-Y.; Zhao, D.-M.; Xiong, X. Q.; Ma, Q. Q.; Cheng, M. S. Chin. J. Org. Chem. 2011, 31, 784 (in Chinese).(李文燕, 赵冬梅, 熊绪琼, 马倩倩, 程卯生, 有机化学, 2011, 31, 784.) (p) Li, Q. H.; Ding, Y.; Yang, X. J. Chin. Chem. Lett. 2014, 25, 1296. (q) Li, Q. H.; Ding, Y.; Huang, N. W. Chin. Chem. Lett. 2014, 25(11), 1469.
[2] Xu, G. Q.; Zhao, Q.; Tang, W. J. Chin. J. Org. Chem. 2014, 34, 1919 (in Chinese).(徐广庆, 赵庆, 汤文军, 有机化学, 2014, 34, 1919.)
[3] Zhao, X. W.; Cui, Y. C. Prog. Chem. 2006, 18, 1652 (in Chinese). (赵晓伟, 崔元臣, 化学进展, 2006, 18, 1652.)
[4] Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419.
[5] For a discussion, see (a) Grushin, V. V.; Alper, H. In Activation of Unreactive Bonds and Organic Synthesis, Ed.: Murai, S., Springer, Berlin, 1999, p. 193.(b) Grushin, V. V.; Alper, H. Chem. Rev. 1994, 94, 1047.
[6] Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. ChemSusChem 2010, 3, 502.
[7] Fihri, A.; Meunier, P.; Hierso, J. C. Coord. Chem. Rev. 2007, 251, 2017.
[8] Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
[9] Liu, L.; Liu, C.; Jin, M. Z. Chin. J. Org. Chem. 2012, 32, 860 (in Chinese).(刘宁, 刘春, 金子林, 有机化学, 2012, 32, 860.)
[10] Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. Tetrahedron Lett. 1986, 27, 279.
[11] Giguere, R. J.; Bray, T. L.; Duncan, S. M.; Majetich, G. Tetrahedron Lett. 1986, 27(41), 4945.
[12] Kappe, C. O. Angew. Chem., Int. Ed. 2004, 43, 6250.
[13] Leadbeater, N. E. Chem. Commun. 2005, 23, 2881.
[14] Dallinger, D.; Kappe, C. O. Chem. Rev. 2007, 107, 2563.
[15] Larhed, M.; Hallberg, A. J. Org. Chem. 1996, 61, 9582.
[16] Blettner. C. G.; König, W. A.; Stenzel, W.; Schotten, T. J. Org. Chem. 1999, 64(11), 3885.
[17] Namboodiri, V. V.; Varma, R. S. Green Chem. 2001, 3(3), 146.
[18] Leadbeater, N. E.; Marco, M. Org. Lett. 2002, 4, 2973.
[19] Leadbeater, N. E.; Marco, M. J. Org. Chem. 2003, 68, 888.
[20] Leadbeater, N. E.; Williams, V. A.; Barnard, T. M.; Collins Jr, M. J. Org. Process Res. Dev. 2006, 10, 833.
[21] Bedford, R. B.; Buts, C. P.; Hurst, T. E.; Lidström, P. Adv. Synth. Catal. 2004, 346, 1627.
[22] Anderson, K. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 2005, 44, 6173.
[23] Genov, M.; Almorín, A.; Espinet, P. Tetrahedron: Asymmetry 2007, 18, 625.
[24] Zhang, Y. Q. J. Chem. Res. 2013, 375.
[25] Kabalka, G. W.; Pagni, R. M.; Wang, L.; Namboodiri, V.; Hair, C. M. Green Chem. 2000, 3, 120.
[26] Kabalka, G. W.; Wang, L.; Pagni, R. M.; Maxwell, H. C.; Vasudevan, N. Synthesis 2003, 217.
[27] Villemin, D.; Caillot, F. Tetrahedron Lett. 2001, 42, 639.
[28] Basu, B.; Das, P.; Bhuiyan, M. M. H.; Jha, S. Tetrahedron Lett. 2003, 44(19), 3817.
[29] Melucci, M.; Barbarella, G.; Sotgiu, G. J. Org. Chem. 2002, 67, 8877.
[30] Sotgiu, G.; Zambianchi, M.; Barbarella, G.; Botta, C. Tetrahedron 2002, 58, 2245.
[31] Melucci, M.; Barbarella, G.; Zambianchi, M.; Pietro, P. D.; Bongini, A. J. Org. Chem. 2004, 69, 4821.
[32] Saha, P.; Naskar, S.; Paira, P.; Hazra, A.; Sahu, K. B.; Paira, R.; Banerjee, S.; Mondal, N. B. Green Chem. 2009, 11, 931.
[33] Cargill, M. R.; Sandford, G.; Tadeusiak, A. J.; Yufit, D. S.; Howard, J. A. K.; Kilickiran, P.; Nelles, G. J. Org. Chem. 2010, 75, 5860.
[34] Gao, F. F.; Liu, F. J.; Lin, C.; Li, W. T.; Wang, S. G.; Qi, C. Z. Catal. Commun. 2013, 5, 27.
[35] Wang, Y.; Sauer, D. R. Org. Lett. 2004, 6, 2793.
[36] Bai, L.; Zhang, Y. M.; Wang, J. X. QSAR Comb. Sci. 2004, 23, 875.
[37] Liu, Y. B.; Khemtong, C.; Hu, J. Chem. Commun. 2004, 398.
[38] Hu, J.; Liu, Y. B. Langmuir 2005, 21, 2121.
[39] Sharma, A. K.; Gowdahalli, K.; Krzeminski, J.; Amin, S. J. Org. Chem. 2007, 72, 8987.
[40] De Souza, A. L. F.; Da Silva, L. C.; Oliveira, B. L.; Antunes, O. A. C. Tetrahedron Lett. 2008, 49, 3895.
[41] Moussa, S.; Siamaki, A. R.; Gupton, B. F.; El-Shall, M. S. ACS Catal. 2012, 2, 145.
[42] Camp, J. E.; Dunsford, J. J.; Cannons, E. P.; Restorick, W. J.; Gadzhieva, A.; Fay, M. W.; Smith, R. J. ACS Sustainable Chem. Eng. 2014, 2, 500.
[43] Gómez-Martínez, M.; Buxaderas, E.; Pastor, I. M.; Alonso, D. A. J. Mol. Catal. A: Chem. 2015, 404~405, 1.
[44] Heidenreich, R. G.; Köhler, K.; Krauter, J. G. E.; Pietsch, J. Synlett 2002, 7, 1118.
[45] Lu, G.; Franzén, R.; Zhang, Q.; Xu, Y. J. Tetrahedron Lett. 2005, 46, 4255.
[46] Arvela, R. K.; Leadbeater, N. E. Org. Lett. 2005, 7, 2101.
[47] Schmidt, B.; Riemer, M.; Karras, M. J. Org. Chem. 2013, 78, 8680.
[48] Schmidt, B.; Riemer, M. J. Org. Chem. 2014, 79, 4104.
[49] Schmidt, B.; Riemer, M. Eur. J. Org. Chem. 2015, 17, 3760.
[50] Al-Amin, M.; Akimoto, M.; Tameno, T.; Ohki, Y.; Takahashi, N.; Hoshiya, N.; Shuto, S.; Arisawa, M. Green Chem. 2013, 15, 1142.
[51] Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S. P. J. Org. Chem. 2004, 69, 3173.
[52] Nun, P.; Martinez, J.; Lamaty, F. Synlett 2009, 1761.
[53] Y?lmaz, Ü.; Küçükbay, H.; ?ireci, N.; Akkurt, M.; Günal, S.; Durmaz, R.; Tahir, M. N. Appl. Organomet. Chem. 2011, 25, 366.
[54] Miao, G. B.; Ye, P.; Yu, L. B.; Baldino, C. M. J. Org. Chem. 2005, 70, 2332.
[55] Solodenko, W.; Schön, U.; Messinger, J.; Glinschert, A.; Kirschning, A. Synlett 2004, 1699.
[56] Kopylovich, M. N.; Lasri, J.; Guedes da Silva, M. F. C.; Pombeiro, A. J. L. Dalton Trans. 2009, 16, 3074.
[57] Cívicos, J. F.; Alonso, D. A.; Nájera, C. Adv. Synth. Catal. 2011, 353, 1683.
[58] Dawood, K. M. Tetrahedron 2007, 63, 9642.
[59] Kostas, I. D.; Heropoulos, G. A.; Kovala-Demertzi, D.; Yadav, P. N.; Jasinski, J. P.; Demertzis, M. A.; Andreadaki, F. J.; Vo-Thanh, G.; Petit, A.; Loupy, A. Tetrahedron Lett. 2006, 47, 4403.
[60] Villemin, D.; Escalonilla, M. J. G.; Saint-Clair, J. F. Tetrahedron Lett. 2001, 42(4), 635.
[61] Qu, G. R.; Xin, P. Y.; Niu, H. Y.; Jin, X.; Guo, X. T.; Yang, X. N.; Guo, H. M. Tetrahedron 2011, 67, 9099.
[62] Zhang, W.; Chen, C. H. T.; Lu, Y. M.; Nagashima, T. Org. Lett. 2004, 6, 1473.
[63] Cívicos, J. F.; Alonso, D. A.; Nájera, C. Adv. Synth. Catal. 2012, 354, 2771.
[64] Seganish, W. M.; DeShong, P. J. Org. Chem. 2004, 69, 1137.
[65] Seganish, W. M.; DeShong, P. Org. Lett. 2004, 6, 4379.
[66] Hadebe, S. W.; Sithebe, S.; Robinson, R. S. Tetrahedron 2011, 67, 4277.
[67] Kabalka, G. W.; Al Masum, M. Tetrahedron Lett. 2005, 46, 6329.
[68] Arvela, R. K.; Leadbeater, N. E.; Mack, T. L.; Kormos, C. M. Tetrahedron Lett. 2006, 47, 217.
[69] Kabalka, G. W.; Zhou, L. L.; Naravane, A. Tetrahedron Lett. 2006, 47, 6887.
[70] Harker, R. L.; Crouch, R. D. Synthesis 2007, 25.
[71] Ahn, H. R.; Cho, Y. A.; Kim, D. S.; Chin, J. G.; Gyoung, Y. S.; Lee, S.; Kang, H.; Ham, J. Org. Lett. 2009, 11, 361.
[72] Kim, D. S.; Ham, J. Org. Lett. 2010, 12, 1092.
[73] Nehls, B. S.; Asawapirom, U.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Adv. Funct. Mater. 2004, 14, 352.
[74] Nehls, B. S.; Fldner, S.; Preis, E.; Farrell, T.; Scherf, U. Macromolecules 2005, 38, 687.
[75] Glasnov, T. N.; Stadlbauer, W.; Kappe, C. O. J. Org. Chem. 2005, 70, 3864.
[76] Hashim, J.; Glasnov, T. N.; Kremsner, J. M.; Kappe, C. O. J. Org. Chem. 2006, 71, 1707.
[77] Mathews, C. J.; Taylor, J.; Tyte, M. J.; Worthington, P. A. Synlett 2005, 538.
[78] Song, Y. S.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2005, 46, 5987.
[79] Song, Y. S.; Lee, Y. J.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2006, 47, 7427.
[80] Heo, Y.; Song, Y. S.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2006, 47, 3091.
[81] Arimitsu, S.; Jacobsen, J. M.; Hammond, G. B. J. Org. Chem. 2008, 73, 2886.
[82] Cao, P.; Qu, J. Y.; Burton, G.; Rivero, R. A. J. Org. Chem. 2008, 73, 7204.
[83] Crozet, M. D.; Zink, L.; Remusat, V.; Curti, C.; Vanelle, P. Synthesis 2009, 3150.
[84] Cohen, A.; Crozet, M. D.; Rathelot, P.; Vanelle, P. Green Chem. 2009, 11, 1736.
[85] St. Jean, Jr., D. J.; Poon, S. F.; Schwarzbach, J. L. Org. Lett. 2007, 9, 4893.
[86] Vishnumurthy, K.; Makriyannis, A. J. Comb. Chem. 2010, 12, 664.
[87] Kabri, Y.; Gellis, A.; Vanelle, P. Eur. J. Org. Chem. 2009, 4059.
[88] Kabri, Y.; Crozet, M. D.; Terme, T.; Vanelle, P. Eur. J. Org. Chem. 2015, 3806.
[89] Appukkuttan, P.; Dehaen, W.; Van der Eycken, E. Org. Lett. 2005, 7, 2723.
[90] Appukkuttan, P.; Dehaen, W.; Van der Eycken, E. Chem. Eur. J. 2007, 13, 6452.
[91] Lépine, R.; Zhu, J. Org. Lett. 2005, 7, 2981.
[92] Wannberg, J.; Sabnis, Y. A.; Vrang, L.; Samuelsson, B.; Karlén, A.; Hallberg, A.; Larhed, M. Bioorg. Med. Chem. 2006, 14, 5303.
[93] Ng-Choi, I.; Soler, M.; Cerezo, V.; Badosa, E.; Montesinos, E.; Planas, M.; Feliu, L. Eur. J. Org. Chem. 2012, 4321.
[94] Benali, O.; Deal, M.; Farrant, E.; Tapolczay, D.; Wheeler, R. Org. Process Res. Dev. 2008, 12, 1007.
[95] De Clercq, E. Nat. Rev. Drug Discovery2002, 1, 13.
[96] De Clercq, E. Antiviral Res. 2005, 67, 56.
[97] Capek, P.; Pohl, R.; Hocek, M. Org. Biomol. Chem. 2006, 4, 2278.
[98] Western, E. C.; Shaughnessy, K. H. J. Org. Chem. 2005, 70, 6378.
[99] Western, E. C.; Daft, J. F.; Johnson, E. M.; Gannett, P. M.; Shaughnessy, K. H. J. Org. Chem. 2003, 68, 6767.
[100] Zhu, R.; Qu, F.; Quéléver, G.; Peng, L. Tetrahedron Lett. 2007, 48, 2389.
[101] Gallagher-Duval, S.; Hervé, G.; Sartori, G.; Enderlin, G.; Len, C. New J. Chem. 2013, 37, 1989.
[102] Lussier, T.; Hervé, G.; Enderlin, G.; Len, C. RSC Adv. 2014, 4, 46218.
[103] Spencer, J.; Baltus, C. B.; Patel, H.; Press, N. J.; Callear, S. K.; Male, L.; Coles. S. J. ACS Comb. Sci. 2011, 13, 24.
[104] Arisawa, M.; Sato, T.; Hoshiya, N.; Al-Amin, M.; Kogami, Y.; Shuto, S. ACS Comb. Sci. 2014, 16, 215.
[105] Nakagawa, K.; Hisamatsu, Y.; Moromizato, S.; Kohno, M.; Aoki, S. Inorg. Chem. 2014, 53, 409.
[106] Konda, V.; Rydfjord, J.; Sävmarker, J.; Larhed, M. Org. Process Res. Dev. 2014, 18, 1413.
[107] (a) Baghbanzadeh, M.; Pilger, C.; Kappe, C. O. J. Org. Chem. 2011, 76, 1507; (b)Singh, N.; Singh, R.; Raghuvanshi, D. S.; Singh, K. N. Org. Lett. 2013, 15, 5874; (c) Singh, K. B.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Der Eycken, E. V. Org. Lett. 2006, 8, 1863.
/
| 〈 |
|
〉 |