

Chinese Journal of Organic Chemistry >
A Novel Naphthalimide-Rhodamine Fluorescence Sensor: Synthesis, Aggregation-Induced Emission Enhancement and Its Dual-Channel Detection Property
Received date: 2015-06-17
Revised date: 2015-09-06
Online published: 2015-09-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 61178057).
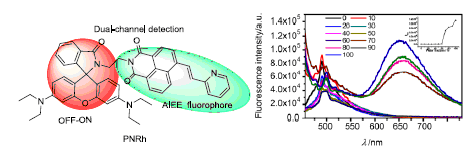
A novel fluorescence probe based on naphthalimide-rhodamine N,N'-rhodamine acyl ethylamino-4-pyridine vinyl-1,8-naphathlimide (PNRh) was synthesized and characterized by 1H NMR, 13C NMR and HRMS techniques. Fluorescence spectra of PNRh in solid, ethanol/water mixed solution were measured. The results demonstrated that PNRh performed luminous yellow fluorescence in the solid state and the maximum emission wavelength was 592 nm. With the increasing of water content, the fluorescence intensity was enhanced in the ethanol/water mixed solution. When the water content reached 100%, the fluorescence intensity got the highest, and the color of the solution turned from slight blue fluorescence to kermesinus fluorescence. In addition, metal ion probe behavior of PNRh was detected. In a word, PNRh, possessed the characteristic of aggregation-induced emission enhancement (AIEE), was a dual-channel fluorescence sensor for detection Hg2+ and Cr3+ ions.
Sun Jingfu , Qian Ying . A Novel Naphthalimide-Rhodamine Fluorescence Sensor: Synthesis, Aggregation-Induced Emission Enhancement and Its Dual-Channel Detection Property[J]. Chinese Journal of Organic Chemistry, 2016 , 36(1) : 151 -157 . DOI: 10.6023/cjoc201506022
[1] Ton, X. A.; Acha, V.; Bonomi, P.; Bui, B. T. S.; Haupt, K. Biosens. Bioelectron. 2015, 64, 359.
[2] Jia, T.; Fu, C.-Y.; Huang, C.-S.; Yang, H.-T.; Jia, N.-Q. ACS Appl. Mater. Interfaces 2015, 7, 10013.
[3] Li, K.-B.; Wang, H.; Zang, Y.; He, X.-P.; Li, J.; Chen, G.-R.; Tian. H. ACS Appl. Mater. Interfaces 2014, 6, 19600.
[4] Yu, C.-W.; Zhang, J.; Li, J.-H.; Liu, P.; Wei, P.-H.; Chen, L.-X. Microchim. Acta 2011, 174, 247.
[5] Tang, L.-J.; Li, F.-F.; Liu, M.-H.; Raju, N. Spectrochim. Acta, Part A 2011, 78, 1168.
[6] Quang, D. T.; Kim, J. S. Chem. Rev. 2010, 110, 6280.
[7] Seo, H.; Mi, E. J.; Ranganathan, K.; Lee, K. H.; Kim, K. T.; Lim, W.; Rhee, Y. M.; Ahn, K. H. Org. Lett. 2014, 16, 1374.
[8] Sun, S.-G.; Qiao, B.; Jiang, N.; Wang, J.-T.; Zhang, S.; Peng, X.-J. Org. Lett.2014, 16, 1132.
[9] Lou, X.-D.; Qiang, L.; Qin, J.-G.; Li, Z. ACS Appl. Mater. Interfaces 2009, 1, 2529.
[10] She, M.-Y.; Yang, Z.; Yin, B.; Zhang, J.; Gu, J.; Yin, W.-T.; Li, J.-L.; Zhao, G.-F.; Shi, Z. Dyes Pigm.2012, 92, 1337.
[11] Bao, Y.-Y.; Keersmaecker, H. D.; Corneillie, S.; Yu, F.; Mizuno, H.; Zhang, G.-F.; Hofkens, J.; Mendrek, B.; Kowalczuk, A.; Smet, M. Chem. Mater. 2015, 27, 3450.
[12] Lan, H.-C.; Wen, Y.; Shi, Y.-M.; Liu, K.-Y.; Mao, Y.-Y.; Yi, T. Analyst2014, 139, 5223.
[13] Yang, Z.; She, M.-Y.; Yin, B.; Cui, J.-H.; Zhang, Y.-Z.; Sun, W.; Li, J.-L.; Shi, Z. J. Org. Chem.2011, 77, 1143.
[14] Saha, S.; Mahato, P.; Upendar, R. G.; Suresh, E.; Chakrabarty, A.; Baidya, M.; Ghosh, S. K.; Das, A. Inorg. Chem. 2011, 51, 336.
[15] Huang K.-W.; Yang H.; Zhou Z.-G; Yu, M.-X.; Li, F.-Y.; Gao, X.; Yi, T.; Huang, C.-H. Org. Lett. 2008, 10, 2557.
[16] Fan, J.-L.; Zhan, P.; Hu, M.-M.; Sun, W.; Tang, J.-Z.; Wang, J.-Y.; Sun, S.-G.; Song, F.-L.; Peng, X.-J. Org. Lett. 2013, 15, 492.
[17] Mahato, P.; Saha, S.; Suresh, E.; Liddo, R. D.; Parnigotto, P. P.; Conconi, M. T.; Kesharwani, M. K.; Ganguly, B.; Das, A. Inorg. Chem. 2012, 51, 1769.
[18] Hu F.-Z.; Zheng, B.; Wang, D.-M.; Liu, M.-P.; Du, J.; Xiao, D. Analyst 2014, 139, 3607.
[19] Georgiev, N. I.; Asiri, A. M.; Qusti, A. H.; Alamry, K. A.; Bojinov, V. B. Dyes Pigm.2014, 102, 35.
[20] Kumar, M.; Kumar, N.; Bhalla, V.; Singh, H.; Sharma, P. R.; Kaur, T. Org. Lett. 2011, 13, 1422.
[21] Tian, Z.-Y.; Su, L.-P.; Xie, S.-Q.; Zhao, J.; Wang, C.-J. Chin. J. Org. Chem. 2013, 33, 1514 (in Chinese).(田智勇, 苏雷朋, 谢松强, 赵瑾, 王超杰, 有机化学, 2013, 33, 1514.)
[22] Zhang, F.; Tan, Z.; Yan, B.-R.; Pan, D.-W.; Bao, X.-P. Chin. J. Org. Chem. 2014, 34, 2499 (in Chinese).(张峰, 谭赞, 闫柏任, 潘顶伍, 鲍小平, 有机化学, 2014, 34, 2499.)
[23] Luo, X.-Y.; Qian, Y. Chin. J. Org. Chem. 2013, 33, 2423 (in Chinese.) (罗晓燕, 钱鹰, 有机化学, 2013, 33, 2423.)
[24] Meng, W.-F.; Yang, M.-P.; Cheng, Z.; Li, S.-N.; Yang, B.-Q. Chin. J. Org. Chem. 2014, 34, 398 (in Chinese).(孟文斐, 杨美盼, 成昭, 李少妮, 杨秉勤, 有机化学, 2014, 34, 398.)
[25] Hong, Y.-N.; Lam, J. W. Y.; Tang, B.-Z. Chem. Soc. Rev. 2011, 40, 5361.
[26] Mei, J.; Hong, Y.-N.; Lam, J. W. Y.; Qin, A.; Tang, Y.; Tang, B.-Z. Adv. Mater. 2014, 26, 5429.
[27] Zhao, Z.-J.; Lam, J. W. Y.; Tang, B.-Z. J. Mater. Chem. 2012, 22, 23726.
[28] Yu, G.; Yin, S.-W.; Liu, Y.-Q.; Chen, J.-S.; Xu, X.-J.; Sun, X.-B.; Ma, D.; Zhan, X.-W.; Peng, Q.; Shuai, Z.-G.; Tang, B.-Z.; Zhu, D.-B.; Fang, W.-W.; Luo, Y. J. Am. Chem. Soc. 2005, 127, 6335.
[29] Sun, J.-F.; Qian, Y. Chin. J. Org. Chem. 2015, 35, 1104 (in Chinese).(孙京府, 钱鹰, 有机化学, 2015, 35,1104.)
[30] Liu, Y.; Tao, X.-T.; Wang, F.-Z.; Dang, X.-N.; Zou, D.-C.; Ren, Y.; Jiang, M.-H. J. Phys. Chem. C2008, 112, 3975.
[31] Liu, Y.; Tao, X.-T.; Wang, F.-Z.; Dang, X.-N.; Zou, D.-C.; Ren, Y.; Jiang, M.-H. J. Phys. Chem. C2007, 111, 6544.
[32] Silva, A. P. D.; Gunaratne, H. Q. N.; Habib-Jiwan, J. L.; Mccoy, C. P.; Rice, T. E.; Soumillion, J. P. Angew. Chem., Int. Ed.1995, 34, 1728.
[33] Zhang, X.; Shiraishi, Y.; Hirai, T. Org. Lett. 2007, 9, 5039.
/
| 〈 |
|
〉 |